Chapter 16
1. Two molecules of ATP are produced per molecule of glyceraldehyde 3-
2. (a) 4; (b) 3; (c) 1; (d) 6; (e) 8; (f) 2; (g) 10; (h) 9; (i) 7; (j) 5.
3. In both cases, the electron donor is glyceraldehyde 3-
4. (a) 3 ATP; (b) 2 ATP; (c) 2 ATP; (d) 2 ATP; (e) 4 ATP.
5. Glucokinase enables the liver to remove glucose from the blood when hexokinase is saturated, ensuring that glucose is captured for later use.
6. The GAP formed is immediately removed by subsequent reactions, resulting in the conversion of DHAP into GAP by the enzyme.
7. A thioester couples the oxidation of glyceraldehyde 3-
1,3-
8. Glycolysis is a component of alcoholic fermentation, the pathway that produces alcohol for beer and wine. The belief was that understanding the biochemical basis of alcohol production might lead to a more-
9. The conversion of glyceraldehyde 3-
10. Glucose 6-
11. The energy needs of a muscle cell vary widely, from rest to intense exercise. Consequently, the regulation of phosphofructokinase by energy charge is vital. In other tissues, such as the liver, ATP concentration is less likely to fluctuate and will not be a key regulator of phosphofructokinase.
12. (a) 6; (b) 1; (c) 7; (d) 3; (e) 2; (f) 5 ; (g) 4.
13. The ΔG°′ for the reverse of glycolysis is +90 kJ mol−1 (+22 kcal mol−1), far too endergonic to take place.
14. The conversion of glucose into glucose 6-
15. Lactic acid is a strong acid. If it remained in the cell, the pH of the cell would fall, which could lead to the denaturation of muscle protein and result in muscle damage.
16. GLUT2 transports glucose only when the blood concentration of glucose is high, which is precisely the condition in which the β cells of the pancreas secrete insulin.
17.
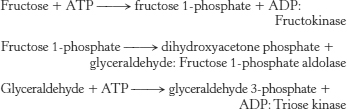
The primary controlling step of glycolysis catalyzed by phosphofructokinase is bypassed by the preceding reactions. Glycolysis will proceed in an unregulated fashion.
18. (a) A, B; (b) C, D; (c) D; (d) A; (e) B; (f) C; (g) A; (h) D; (i) none; (j) A; (k) A.
19. (a) 4; (b) 10; (c) 1; (d) 5; (e) 7; (f) 8; (g) 9; (h) 2; (i) 3; (j) 6.
20. Without triose isomerase, only one of the two three-
21. Glucose is reactive because its open-
22. (a) The label is in the methyl carbon atom of pyruvate. (b) 5 mCi mM−1. The specific activity is halved because the number of moles of product (pyruvate) is twice that of the labeled substrate (glucose).
23. (a) Glucose + 2 Pi + 2 ADP → 2 lactate + 2 ATP.
(b) ΔG = −114 kJ mol−1 (−27.2 kcal mol−1).
24. 3.06 × 10−5
25. The equilibrium concentrations of fructose 1,6-
26. All three carbon atoms of 2,3-
A20
27. Hexokinase has a low ATPase activity in the absence of a sugar because it is in a catalytically inactive conformation. The addition of xylose closes the cleft between the two lobes of the enzyme. However, xylose lacks a hydroxymethyl group, and so it cannot be phosphorylated. Instead, a water molecule at the site normally occupied by the C-
28. (a) The fructose 1-
(b) Phosphofructokinase, a key control enzyme, is bypassed.
29. The reverse of glycolysis is highly endergonic under cellular conditions. The expenditure of six NTP molecules in gluconeogenesis renders gluconeogenesis exergonic.
30. (a) 2, 3, 6, 9; (b) 1, 4, 5, 7, 8.
31. Lactic acid is capable of being further oxidized and is thus useful energy. The conversion of this acid into glucose saves the carbon atoms for future combustion.
32. In glycolysis, the formation of pyruvate and ATP by pyruvate kinase is irreversible. This step is bypassed by two reactions in gluconeogenesis: (1) the formation of oxaloacetate from pyruvate and CO2 by pyruvate carboxylase and (2) the formation of phosphoenolpyruvate from oxaloacetate and GTP by phosphoenolpyruvate carboxykinase. The formation of fructose 1,6-
33. Reciprocal regulation at the key allosteric enzymes in the two pathways. For instance, PFK is stimulated by fructose 2,6-
34. Muscle is likely to produce lactic acid during contraction. Lactic acid is a strong acid and cannot accumulate in muscle or blood. Liver removes the lactic acid from the blood and converts it into glucose. The glucose can be released into the blood or stored as glycogen for later use.
35. Glucose produced by the liver could not be released into the blood. Tissues that rely on glucose as an energy source would not function as well unless glucose was provided in the diet.
36. Glucose is an important energy source for both tissues and is essentially the only energy source for the brain. Consequently, these tissues should never release glucose. Glucose release is prevented by the absence of glucose 6-
37. 6 NTP (4 ATP and 2 GTP); 2 NADH.
38. (a) None; (b) none; (c) 4 (2 ATP and 2 GTP); (d) none.
39. If the amino groups are removed from alanine and aspartate, the ketoacids pyruvate and oxaloacetate are formed. Both of these molecules are components of the gluconeogenic pathway.
40. (a) Increased; (b) increased; (c) increased; (d) decreased.
41. Fructose 2,6-
42. Reactions in parts b and e would be blocked.
43. There will be no labeled carbons. The CO2 added to pyruvate (formed from the lactate) to form oxaloacetate is lost with the conversion of oxaloacetate into phosphoenolpyruvate.
44. The net reaction in the presence of arsenate is
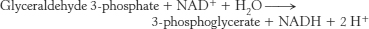
Glycolysis proceeds in the presence of arsenate, but the ATP normally formed in the conversion of 1,3-
45. This example illustrates the difference between the stoichiometric and the catalytic use of a molecule. If cells used NAD+ stoichiometrically, a new molecule of NAD+ would be required each time a molecule of lactate was produced. As we will see, the synthesis of NAD+ requires ATP. On the other hand, if the NAD+ that is converted into NADH could be recycled and reused, a small amount of the molecule could regenerate a vast amount of lactate, which is the case in the cell. NAD+ is regenerated by the oxidation of NADH and reused. NAD+ is thus used catalytically.
46. Consider the equilibrium equation of adenylate kinase:
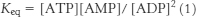
or
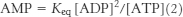
Recall that [ATP] > [ADP] > [AMP] in the cell. As ATP is utilized, a small decrease in its concentration will result in a larger percentage increase in [ADP] because its concentration is greater than that of ADP. This larger percentage increase in [ADP] will result in an even greater percentage increase in [AMP] because the concentration of AMP is related to the square of [ADP]. In essence, equation 2 shows that monitoring the energy status with AMP magnifies small changes in [ATP], leading to tighter control.
47. The synthesis of glucose during intense exercise provides a good example of interorgan cooperation in higher organisms. When muscle is actively contracting, lactate is produced from glucose by glycolysis. The lactate is released into the blood and absorbed by the liver, where it is converted by gluconeogenesis into glucose. The newly synthesized glucose is then released and taken up by the muscle for energy generation.
48. The input of four additional high-
49. The mechanism is analogous to that for triose phosphate isomerase (Figure 16.5). It proceeds through an enediol intermediate. The active site would be expected to have a general base (analogous to Glu 165 in TPI) and a general acid (analogous to His 95 in TPI).
50. Galactose is a component of glycoproteins. Possibly, the absence of galactose leads to the improper formation or function of glycoproteins required in the central nervous system. More generally, the fact that the symptoms arise in the absence of galactose suggests that galactose is required in some fashion.
51. Using the Michaelis–
52. Fructose 2,6-
53. (a) Curiously, the enzyme uses ADP as the phosphoryl donor rather than ATP.
(b) Both AMP and ATP behave as competitive inhibitors of ADP, the phosphoryl donor. Apparently, the P. furiosus enzyme is not allosterically inhibited by ATP.
A21
54. (a) If both enzymes operated simultaneously, the following reactions would take place:
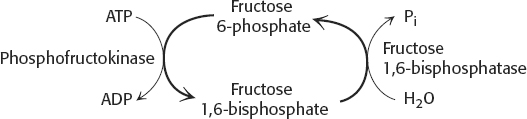
The net result would be simply:
ATP + H2O → ADP + Pi
The energy of ATP hydrolysis would be released as heat.
(b) Not really. For the cycle to generate heat, both enzymes must be functional at the same time in the same cell.
(c) The species B. terrestris and B. rufocinctus might show some futile cycling because both enzymes are active to a substantial degree.
(d) No. These results simply suggest that simultaneous activity of phosphofructokinase and fructose 1,6-
55. ATP initially stimulates PFK activity, as would be expected for a substrate. Higher concentrations of ATP inhibit the enzyme. Although this effect seems counterintuitive for a substrate, recall that the function of glycolysis in muscle is to generate ATP. Consequently, high concentrations of ATP signal that the ATP needs are met and glycolysis should stop. In addition to being a substrate, ATP is an allosteric inhibitor of PFK.