Chapter 26
1. Glycerol 3-
2. Glycerol 3-
3. Glycerol + 4 ATP + 3 fatty acids + 4 H2O → triacylglycerol + ADP + 3 AMP + 7 Pi + 4 H+.
4. Glycerol + 3 ATP + 2 fatty acids + 2 H2O + CTP + ethanolamine → phosphatidylethanolamine + CMP + ADP + 2 AMP + 6 Pi + 3 H+.
5. Three. One molecule of ATP to form phosphorylethanolamine and two molecules of ATP to regenerate CTP from CMP.
6. All are synthesized from ceramide. In sphingomyelin, the terminal hydroxyl group of ceramide is modified with phosphorylcholine. In a cerebroside, the hydroxyl group has a glucose or galactose attached. In a ganglioside, oligosaccharide chains are attached to the hydroxyl group.
7. (i) Activate the diacylglycerol as CDP-
8. (a) CDP-
9. Such mutations are seen in mice. The amount of adipose tissue would decrease severely because diacylglycerol could not be formed. Normally, diacylglycerol is acylated to form triacylglycerols. If there were deficient phosphatidic acid phosphatase activity, no triacylglycerols would form.
10. (a) 8; (b) 4; (c) 1; (d) 9; (e) 3; (f) 10; (g) 5; (h) 2; (i) 6; (j) 7.
11. (i) The synthesis of activated isoprene units (isopentyl pyrophosphate), (ii) the condensation of six of the activated isoprene units to form squalene, and (iii) cyclization of the squalene to form cholesterol.
12. The amount of reductase and its activity control the regulation of cholesterol biosynthesis. Transcriptional control is mediated by SREBP. Translation of the reductase mRNA also is controlled. The reductase itself may undergo regulated proteolysis. Finally, the activity of the reductase is inhibited by phosphorylation by AMP kinase when ATP levels are low.
13. (a and b) None, because the label is lost as CO2.
14. The hallmark of this genetic disease is elevated cholesterol levels in the blood of even young children. The excess cholesterol is taken up by marcrophages, which eventually results in the formation of plaques and heart disease. There are many mutations that cause the disease, but all result in malfunctioning of the LDL receptor.
15. The categories of mutations are: (i) no receptor is synthesized; (ii) receptors are synthesized but do not reach the plasma membrane, because they lack signals for intracellular transport or do not fold properly; (iii) receptors reach the cell surface, but they fail to bind LDL normally because of a defect in the LDL-
16. “None of your business” and “I don’t talk biochemistry until after breakfast” are appropriate but rude and uninformative answers. A better answer might be: “Although it is true that cholesterol is a precursor to steroid hormones, the rest of the statement is oversimplified. Cholesterol is a component of membranes, and membranes literally define cells, and cells make up tissues. But to say that cholesterol ‘makes’ cells and tissues is wrong.”
17. Statins are competitive inhibitors of HMG-
18. No. Cholesterol is essential for membrane function and as a precursor for bile salts and steroid hormones. The complete lack of cholesterol would be lethal.
19. Deamination of cytidine to uridine changes CAA (Gln) into UAA (stop).
20. The LDL contains apolipoprotein B-
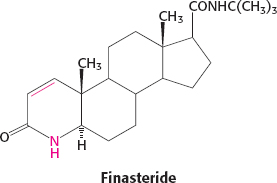
21. Benign prostatic hypertrophy can be treated by inhibiting 5α-reductase. Finasteride, the 4-
22. Patients who are most sensitive to debrisoquine have a deficiency of a liver P450 enzyme encoded by a member of the CYP2 subfamily. This characteristic is inherited as an autosomal recessive trait. The capacity to degrade other drugs may be impaired in people who hydroxylate debrisoquine at a slow rate, because a single P450 enzyme usually handles a broad range of substrates.
A39
23. Many hydrophobic odorants are deactivated by hydroxylation. Molecular oxygen is activated by a cytochrome P450 monooxygenase. NADPH serves as the reductant. One oxygen atom of O2 goes into the odorant substrate, whereas the other is reduced to water.
24. Recall that dihydrotestosterone is crucial for the development of male characteristics in the embryo. If a pregnant woman were to be exposed to Propecia, the 5α-reductase of the male embryo would be inhibited, which could result in severe developmental abnormalities.
25. The oxygenation reactions catalyzed by the cytochrome P450 family permit greater flexibility in biosynthesis. Because plants are not mobile, they must rely on physical defenses, such as thorns, and chemical defenses, such as toxic alkaloids. The larger P450 array might permit greater biosynthetic versatility.
26. This knowledge might enable clinicians to characterize the likelihood of a patient’s having an adverse drug reaction or being susceptible to chemical-
27. The honeybees may be especially sensitive to environmental toxins, including pesticides, because these chemicals are not readily detoxified, owing to the minimal P450 system.
28. A deficiency of the hydroxylase will cause a build-
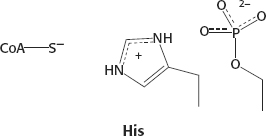
29. The core structure of a steroid is four fused rings: three cyclohexane rings and one cyclopentane ring. In vitamin D, the B ring is split by ultraviolet light.
30. The negatively charged phosphoserine residue interacts with the positively charged protonated histidine residue and decreases its ability to transfer a proton to the thiolate.
31. The methyl group is first hydroxylated. The hydroxymethylamine eliminates formaldehyde to form methylamine.
32. Note that a cytidine nucleotide plays the same role in the synthesis of these phosphoglycerides as a uridine nucleotide does in the formation of glycogen (Section 21.4). In all of these biosyntheses, an activated intermediate (UDP-
33. The attachment of isoprenoid side chains confers hydrophobic character. Proteins having such a modification are targeted to membranes.
34. 3-
35. One way in which phosphatidylcholine can be synthesized is by the addition of three methyl groups to phosphatidylethanolamine. The methyl donor is a modified form of methionine, S-adenosylmethionine or SAM (Section 24.2).
36. Mutations could occur in the gene encoding the sodium channel that would prevent the action of DDT. Alternatively, P450 enzyme synthesis could be increased to accelerate metabolism of the insecticide to inactive metabolites. In fact, both types of responses to insecticides have been observed.
37. Citrate is transported out of the mitochondria in times of plenty. ATP-
38. (a) There is no effect. (b) Because actin is not controlled by cholesterol, the amount isolated should be the same in both experimental groups; a difference would suggest a problem in the RNA isolation. (c) The presence of cholesterol in the diet dramatically reduces the amount of HMG-
39. (a) The activity of the enzyme from the GD patient is the same as that from the control. This suggests that GD is not caused by a mutation that impairs enzyme activity.
(b) There is much less GCase in the GD sample. Because the same number of cells were used in the two samples (confirmed by the identical levels of actin in both samples), we know that the difference in amount of GCase is not due to different quantities of cells. Two possible explanations are that transcription is impaired in the GD sample or that the enzyme in GD is more readily destroyed than the control.
(c) Apparently, the enzyme is being synthesized but then degraded.
(d) The defect in the enzyme from the GD patient seems to result in more rapid degradation of the enzyme by the proteasome. The fact that the amount of GCase present in the control increased upon proteasomal inhibition suggests that GCase may undergo rapid turnover under normal conditions.