Chapter 9
1. For the amide substrate, the formation of the acyl-
2. The histidine residue in the substrate can substitute to some extent for the missing histidine residue of the catalytic triad of the mutant enzyme.
3. No. The catalytic triad works as a unit. After this unit has been made ineffective by the mutation of histidine to alanine, the further mutation of serine to alanine should have only a small effect.
4. The substitution corresponds to one of the key differences between trypsin and chymotrypsin, and so trypsin-
A11
In fact, additional changes are required to effect this specificity change.
5. Imidazole is apparently small enough to reach the active site of carbonic anhydrase and compensate for the missing histidine. Buffers with large molecular components cannot do so, and the effects of the mutation are more evident.
6. No. The odds of such a sequence being present are approximately 1 in 410 = 1,048,576. Because a typical viral genome has only 50,000 bp, the target sequence would be unlikely to be present.
7. No, because the enzyme would destroy the host DNA before protective methylation could take place.
8. No. The bacteria receiving the enzyme would have their own DNA destroyed because they would likely lack the appropriate protective methylase.
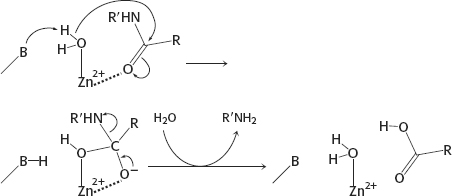
9. EDTA will bind to Zn2+ and remove the ion, which is required for enzyme activity, from the enzyme.
10. (a) The aldehyde reacts with the active-
11. Trypsin.
12. The reaction is expected to be slower by a factor of 10 because the rate depends on the pKa of the zinc-
13. EDTA binds the magnesium necessary for the reaction.
14. ATP hydrolysis is reversible within the active site. ATP hydrolysis takes place within the active site with the incorporation of 18O, ATP is re-
15. If the aspartate is mutated, the protease is inactive and the virus will not be viable.
16. Water substitutes for the hydroxyl group of serine 236 in mediating proton transfer from the attacking water and the γ-phosphoryl group.
17. For subtilisin, the catalytic power is approximately 30 s−1/10−8 s−1 = 3 × 109. For carbonic anhydrase (at pH 7), the catalytic power is approximately 500,000 s−1/0.15 s−1 = 3.3 × 106. Subtilisin is the more powerful enzyme by this criterion.
18. The triple mutant still catalyzes the reaction by a factor of approximately 1000-
19. (a) Cysteine protease: The same as Figure 9.8, except that cysteine replaces serine in the active site and no aspartate is present.
(b) Aspartyl protease:
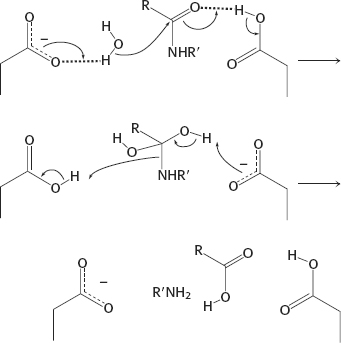
(c) Metalloprotease: