PROBLEMS
Question 12.1
Population density. How many phospholipid molecules are there in a 1-
Question 12.2
Through the looking-
364
Question 12.3
Lipid diffusion. What is the average distance traversed by a membrane lipid in 1 μs, 1 ms, and 1 s? Assume a diffusion coefficient of 10−8 cm2 s−1.
Question 12.4
Protein diffusion. The diffusion coefficient, D, of a rigid spherical molecule is given by
D = kT/6πηr
in which η is the viscosity of the solvent, r is the radius of the sphere, k is the Boltzman constant (1.38 × 10−16 erg degree−1), and T is the absolute temperature. What is the diffusion coefficient at 37° C of a 100-
Question 12.5
Cold sensitivity. Some antibiotics act as carriers that bind an ion on one side of a membrane, diffuse through the membrane, and release the ion on the other side. The conductance of a lipid-
Question 12.6
Melting point 1. Explain why oleic acid (18 carbons, one cis bond) has a lower melting point than stearic acid, which has the same number of carbon atoms but is saturated. How would you expect the melting point of trans-oleic acid to compare with that of cis -oleic acid? Why might most unsaturated fatty acids in phospholipids be in the cis rather than the trans conformation?
Question 12.7
Melting point 2. Explain why the melting point of palmitic acid (C16) is 6.5 degrees lower than that of stearic acid (C18).
Question 12.8
A sound diet. Small mammalian hibernators can withstand body temperatures of 0° to 5°C without injury. However, the body fats of most mammals have melting temperatures of approximately 25°C. Predict how the composition of the body fat of hibernators might differ from that of their nonhibernating cousins.
Question 12.9
Flip-
Lipid vesicles containing phosphatidylserine (98%) and NBD-
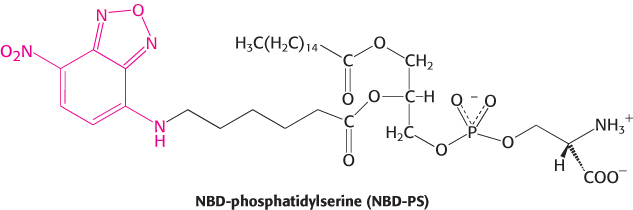
Question 12.10
Flip-
Question 12.11
Linkages. Platelet-
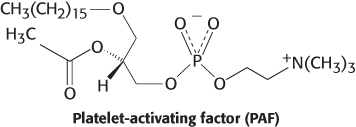
Question 12.12
A question of competition. Would a homopolymer of alanine be more likely to form an α helix in water or in a hydrophobic medium? Explain.
Question 12.13
A false positive. Hydropathy plot analysis of your protein of interest reveals a single, prominent hydrophobic peak. However, you later discover that this protein is soluble and not membrane associated. Explain how the hydropathy plot may have been misleading.
Question 12.14
Maintaining fluidity. A culture of bacteria growing at 37° C was shifted to 25° C. How would you expect this shift to alter the fatty acid composition of the membrane phospholipids? Explain.
Question 12.15
Let me count the ways. Each intracellular fusion of a vesicle with a membrane requires a SNARE protein on the vesicle (called the v-
365
Data Interpretation Problems
Question 12.16
Cholesterol effects. The red curve on the following graph shows the fluidity of the fatty acids of a phospholipid bilayer as a function of temperature. The blue curve shows the fluidity in the presence of cholesterol.
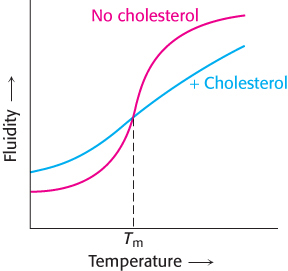
What is the effect of cholesterol?
Why might this effect be biologically important?
Question 12.17
Hydropathy plots. On the basis of the following hydropathy plots for three proteins (A–
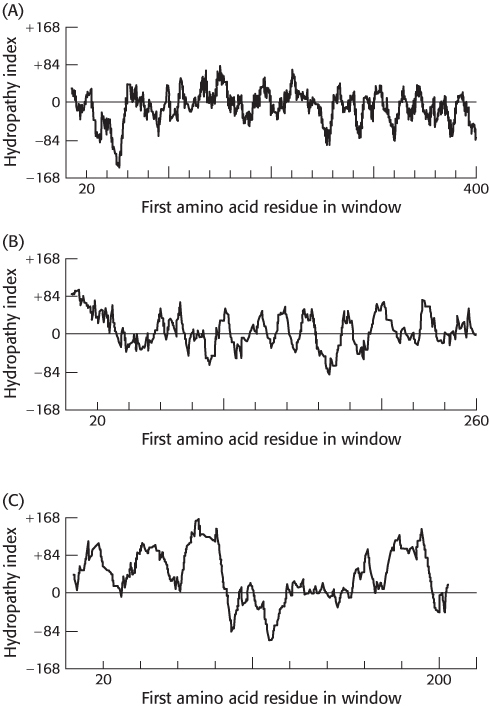
Question 12.18
Not all inhibitors are equal. Ibuprofen and indomethacin are clinically important inhibitors of prostaglandin H2 synthase-
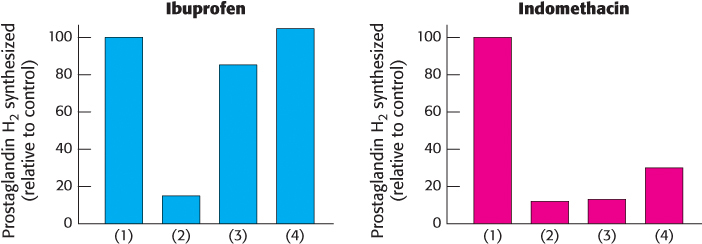
40 min without inhibitor (control)
40 min with inhibitor
40 min with inhibitor, after which the cells were resuspended in medium without inhibitor
40 min with inhibitor, after which the cells were resuspended in medium without inhibitor and incubated for an additional 30 min.
Provide a hypothesis explaining the different results for these two inhibitors.
How would these results look if aspirin were tested in a similar fashion?
Chapter Integration Problem
Question 12.19
The proper environment. An understanding of the structure and function of membrane proteins has lagged behind that of other proteins. The primary reason is that membrane proteins are more difficult to purify and crystallize. Why might this be the case?
366