12.2 There Are Three Common Types of Membrane Lipids
By definition, lipids are water-
Phospholipids are the major class of membrane lipids
Phospholipids are abundant in all biological membranes. A phospholipid molecule is constructed from four components: one or more fatty acids, a platform to which the fatty acids are attached, a phosphate, and an alcohol attached to the phosphate (Figure 12.3). The fatty acid components provide a hydrophobic barrier, whereas the remainder of the molecule has hydrophilic properties that enable interaction with the aqueous environment.
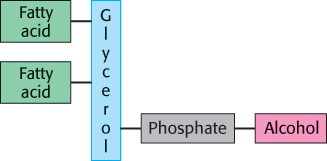
The platform on which phospholipids are built may be glycerol, a three-
In phosphoglycerides, the hydroxyl groups at C-
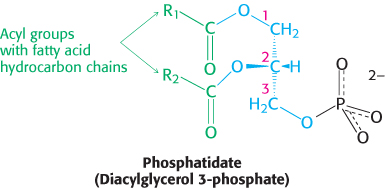
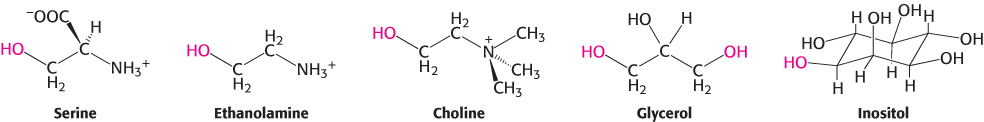
The major phosphoglycerides are derived from phosphatidate by the formation of an ester bond between the phosphate group of phosphatidate and the hydroxyl group of one of several alcohols. The common alcohol moieties of phosphoglycerides are the amino acid serine, ethanolamine, choline, glycerol, and inositol.
345
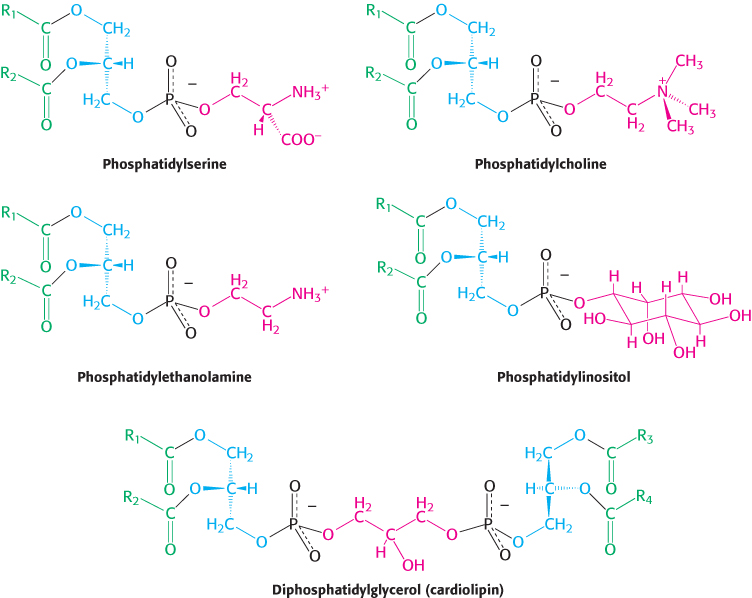
The structural formulas of phosphatidylcholine and the other principal phosphoglycerides—
Sphingomyelin is a phospholipid found in membranes that is not derived from glycerol. Instead, the backbone in sphingomyelin is sphingosine, an amino alcohol that contains a long, unsaturated hydrocarbon chain (Figure 12.6). In sphingomyelin, the amino group of the sphingosine backbone is linked to a fatty acid by an amide bond. In addition, the primary hydroxyl group of sphingosine is esterified to phosphorylcholine.
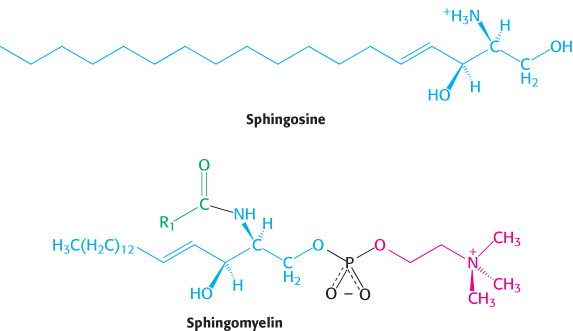
Membrane lipids can include carbohydrate moieties
The second major class of membrane lipids, glycolipids, are sugar-
346
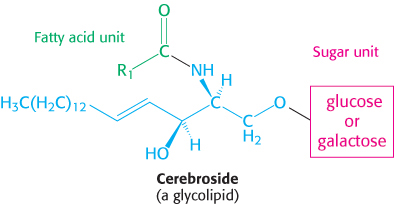
More-
Cholesterol Is a Lipid Based on a Steroid Nucleus
Cholesterol, the third major type of membrane lipid, has a structure that is quite different from that of phospholipids. It is a steroid, built from four linked hydrocarbon rings.
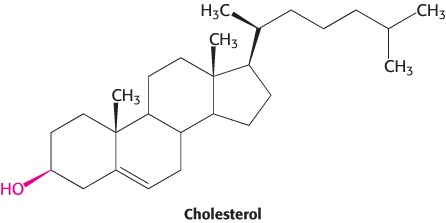
A hydrocarbon tail is linked to the steroid at one end, and a hydroxyl group is attached at the other end. In membranes, the orientation of the molecule is parallel to the fatty acid chains of the phospholipids, and the hydroxyl group interacts with the nearby phospholipid head groups. Cholesterol is absent from prokaryotes but is found to varying degrees in virtually all animal membranes. It constitutes almost 25% of the membrane lipids in certain nerve cells but is essentially absent from some intracellular membranes.
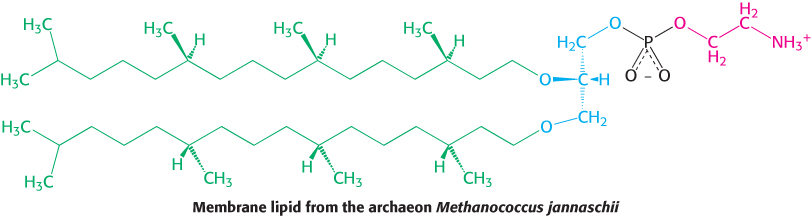
Archaeal membranes are built from ether lipids with branched chains
 The membranes of archaea differ in composition from those of eukaryotes or bacteria in three important ways. Two of these differences clearly relate to the hostile living conditions of many archaea (Figure 12.7). First, the nonpolar chains are joined to a glycerol backbone by ether rather than ester linkages. The ether linkage is more resistant to hydrolysis. Second, the alkyl chains are branched rather than linear. They are built up from repeats of a fully saturated five-
The membranes of archaea differ in composition from those of eukaryotes or bacteria in three important ways. Two of these differences clearly relate to the hostile living conditions of many archaea (Figure 12.7). First, the nonpolar chains are joined to a glycerol backbone by ether rather than ester linkages. The ether linkage is more resistant to hydrolysis. Second, the alkyl chains are branched rather than linear. They are built up from repeats of a fully saturated five-

347
A membrane lipid is an amphipathic molecule containing a hydrophilic and a hydrophobic moiety
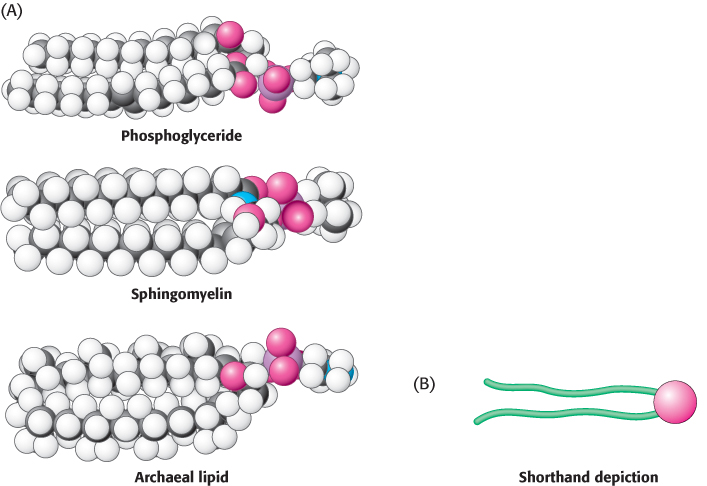
The repertoire of membrane lipids is extensive. However, these lipids possess a critical common structural theme: membrane lipids are amphipathic molecules (amphiphilic molecules), that is, they contain both a hydrophilic and a hydrophobic moiety.
Let us look at a model of a phosphoglyceride, such as phosphatidylcholine. Its overall shape is roughly rectangular (Figure 12.8A). The two hydrophobic fatty acid chains are approximately parallel to each other, whereas the hydrophilic phosphorylcholine moiety points in the opposite direction. Sphingomyelin has a similar conformation, as does the archaeal lipid depicted. Therefore, the following shorthand has been adopted to represent these membrane lipids: the hydrophilic unit, also called the polar head group, is represented by a circle, and the hydrocarbon tails are depicted by straight or wavy lines (Figure 12.8B).
348