12.5 Lipids and Many Membrane Proteins Diffuse Rapidly in the Plane of the Membrane
Biological membranes are not rigid, static structures. On the contrary, lipids and many membrane proteins are constantly in lateral motion, a process called lateral diffusion. The rapid lateral movement of membrane proteins has been visualized by means of fluorescence microscopy using the technique of fluorescence recovery after photobleaching (FRAP; Figure 12.28). First, a cell-
S = (4Dt)12
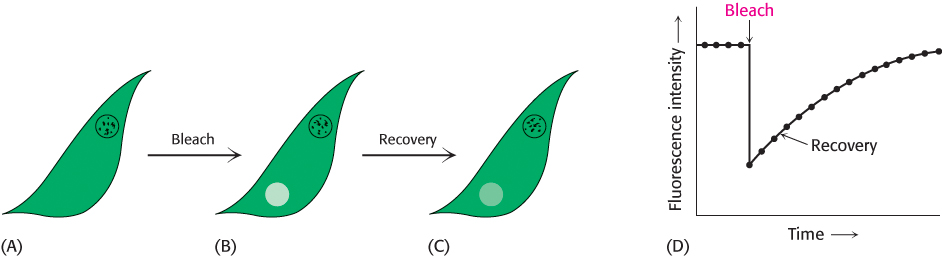
The diffusion coefficient of lipids in a variety of membranes is about 1 μm2 s−1. Thus, a phospholipid molecule diffuses an average distance of 2 μm in 1 s. This rate means that a lipid molecule can travel from one end of a bacterium to the other in a second. The magnitude of the observed diffusion coefficient indicates that the viscosity of the membrane is about 100 times that of water, rather like that of olive oil.
357
In contrast, proteins vary markedly in their lateral mobility. Some proteins are nearly as mobile as lipids, whereas others are virtually immobile. For example, the photoreceptor protein rhodopsin (Section 33.3), a very mobile protein, has a diffusion coefficient of 0.4 μm2 s−1. The rapid movement of rhodopsin is essential for fast signaling. At the other extreme is fibronectin, a peripheral glycoprotein that interacts with the extracellular matrix. For fibronectin, D is less than 10−4 μm2 s−1. Fibronectin has a very low mobility because it is anchored to actin filaments on the inside of the plasma membrane through integrin, a transmembrane protein that links the extracellular matrix to the cytoskeleton.
The fluid mosaic model allows lateral movement but not rotation through the membrane
On the basis of the mobility of proteins in membranes, in 1972 S. Jonathan Singer and Garth Nicolson proposed a fluid mosaic model to describe the overall organization of biological membranes. The essence of their model is that membranes are two-
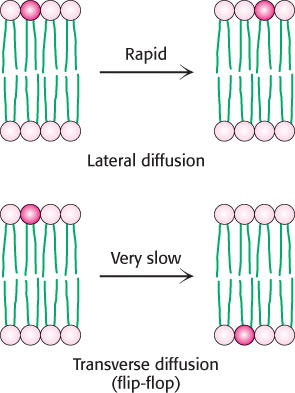
Although the lateral diffusion of membrane components can be rapid, the spontaneous rotation of lipids from one face of a membrane to the other is a very slow process. The transition of a molecule from one membrane surface to the other is called transverse diffusion or flip-
Membrane fluidity is controlled by fatty acid composition and cholesterol content
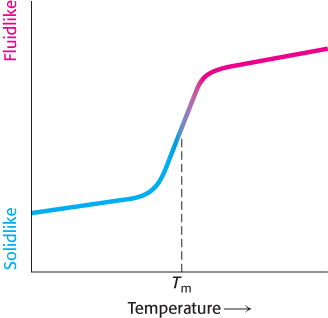
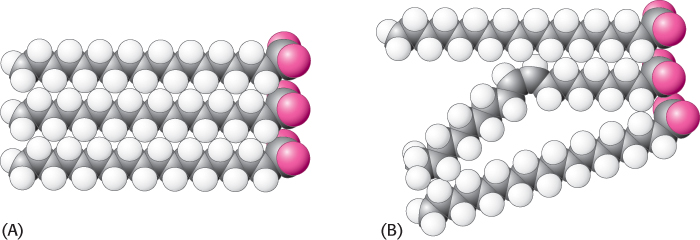
Many membrane processes, such as transport or signal transduction, depend on the fluidity of the membrane lipids, which in turn depends on the properties of fatty acid chains. Fatty acid chains in membrane bilayers can exist in an ordered, rigid state or in a relatively disordered, fluid state. The transition from the rigid to the fluid state takes place abruptly as the temperature is raised above Tm, the melting temperature (Figure 12.30). This transition temperature depends on the length of the fatty acid chains and on their degree of unsaturation (Table 12.3). The presence of saturated fatty acid residues favors the rigid state because their straight hydrocarbon chains interact very favorably with one another. On the other hand, a cis double bond produces a bend in the hydrocarbon chain. This bend interferes with a highly ordered packing of fatty acid chains, and so Tm is lowered (Figure 12.31). The length of the fatty acid chain also affects the transition temperature. Long hydrocarbon chains interact more strongly than do short ones. Specifically, each additional  CH2
CH2 group makes a favorable contribution of about −2 kJ mol−1 (−0.5 kcal mol−1) to the free energy of interaction of two adjacent hydrocarbon chains.
group makes a favorable contribution of about −2 kJ mol−1 (−0.5 kcal mol−1) to the free energy of interaction of two adjacent hydrocarbon chains.
|
Fatty acid |
|||||
|---|---|---|---|---|---|
|
Number of carbons |
Number of double bonds |
Common name |
Systematic name |
Tm (°C) |
|
|
22 |
0 |
Behenate |
n-Docosanote |
75 |
|
|
18 |
0 |
Stearate |
n-Octadecanoate |
58 |
|
|
16 |
0 |
Palmitate |
n-Hexadecanoate |
41 |
|
|
14 |
0 |
Myristate |
n-Tetradecanoate |
24 |
|
|
18 |
1 |
Oleate |
cis-Δ9-Octadecenoate |
−22 |
|
358
Bacteria regulate the fluidity of their membranes by varying the number of double bonds and the length of their fatty acid chains. For example, the ratio of saturated to unsaturated fatty acid chains in the E. coli membrane decreases from 1.6 to 1.0 as the growth temperature is lowered from 42°C to 27°C. This decrease in the proportion of saturated residues prevents the membrane from becoming too rigid at the lower temperature.
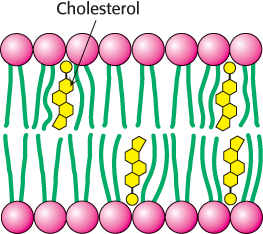
In animals, cholesterol is the key regulator of membrane fluidity. Cholesterol contains a bulky steroid nucleus with a hydroxyl group at one end and a flexible hydrocarbon tail at the other end. Cholesterol inserts into bilayers with its long axis perpendicular to the plane of the membrane. The hydroxyl group of cholesterol forms a hydrogen bond with a carbonyl oxygen atom of a phospholipid head group, whereas the hydrocarbon tail of cholesterol is located in the nonpolar core of the bilayer. The different shape of cholesterol compared with that of phospholipids disrupts the regular interactions between fatty acid chains (Figure 12.32).
Lipid rafts are highly dynamic complexes formed between cholesterol and specific lipids
In addition to its nonspecific effects on membrane fluidity, cholesterol can form specific complexes with lipids that contain the sphingosine backbone, including sphingomyelin and certain glycolipids, and with GPI-
All biological membranes are asymmetric
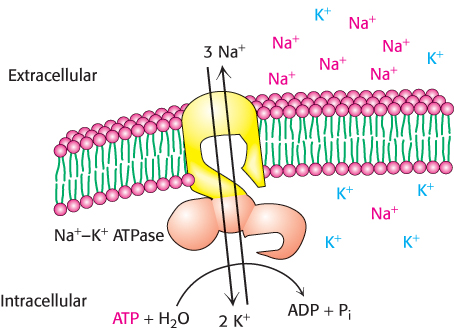
Membranes are structurally and functionally asymmetric. The outer and inner surfaces of all known biological membranes have different components and different enzymatic activities. A clear-
359
Membrane proteins have a unique orientation because, after synthesis, they are inserted into the membrane in an asymmetric manner. This absolute asymmetry is preserved because membrane proteins do not rotate from one side of the membrane to the other and because membranes are always synthesized by the growth of preexisting membranes. Lipids, too, are asymmetrically distributed as a consequence of their mode of biosynthesis, but this asymmetry is usually not absolute, except for glycolipids. In the red-