PROBLEMS
Question 13.1
A helping hand. Differentiate between simple diffusion and facilitated diffusion.
Question 13.2
Powering movement. What are the two forms of energy that can power active transport?
Question 13.3
Carriers. Name the three types of carrier proteins. Which of these can mediate secondary active transport?
Question 13.4
The price of extrusion. What is the free-
Question 13.5
Equilibrium potentials. For a typical mammalian cell, the intracellular and extracellular concentrations of the chloride ion (Cl−) are 4 mM and 150 mM, respectively. For the calcium ion (Ca2+), the intracellular and extracellular concentrations are 0.2 μM and 1.8 mM, respectively. Calculate the equilibrium potentials at 37°C for these two ions.
394
Question 13.6
How sweet it is. Some animal cells take up glucose by a symporter powered by the simultaneous entry of Na+. The entry of Na+ provides a free-
Question 13.7
Variations on a theme. Write a detailed mechanism for transport by the Na+–K+ ATPase based on analogy with the mechanism of the Ca2+ ATPase shown in Figure 13.5.
Question 13.8
Pumping protons. Design an experiment to show that the action of lactose permease can be reversed in vitro to pump protons.
Question 13.9
Opening channels. Differentiate between ligand-
Question 13.10
Different directions. The K+ channel and the Na+ channel have similar structures and are arranged in the same orientation in the cell membrane. Yet the Na+ channel allows sodium ions to flow into the cell and the K+ channel allows potassium ions to flow out of the cell. Explain.
Question 13.11
Differing mechanisms. Distinguish the mechanisms by which uniporters and channels transport ions or molecules across the membrane.
Question 13.12
Short circuit. Carbonyl cyanide 4-
Question 13.13
Working together. The human genome contains more than 20 connexin-
Question 13.14
Structure–
Question 13.15
Hot stuff. When SERCA is incubated with [γ-32P]ATP (a form of ATP in which the terminal phosphate is labeled with radioactive 32P) and calcium at 0°C for 20 seconds and analyzed by gel electrophoresis, a radioactive band is observed at the molecular weight corresponding to full-
Question 13.16
A dangerous snail. Cone snails are carnivores that inject a powerful set of toxins into their prey, leading to rapid paralysis. Many of these toxins are found to bind to specific ion-
Question 13.17
Pause for effect. Immediately after the repolarization phase of an action potential, the neuronal membrane is temporarily unable to respond to the stimulation of a second action potential, a phenomenon referred to as the refractory period. What is the mechanistic basis for the refractory period?
Question 13.18
Only a few. Why do only a small number of sodium ions need to flow through the Na+ channel to change the membrane potential significantly?
Question 13.19
More than one mechanism. How might a mutation in a cardiac voltage-
Question 13.20
Mechanosensitive channels. Many species contain ion channels that respond to mechanical stimuli. On the basis of the properties of other ion channels, would you expect the flow of ions through a single open mechanosensitive channel to increase in response to an appropriate stimulus? Why or why not?
Question 13.21
Concerted opening. Suppose that a channel obeys the concerted allosteric model (MWC model, Section 7.2). The binding of ligand to the R state (the open form) is 20 times as tight as that to the T state (the closed form). In the absence of ligand, the ratio of closed to open channels is 105. If the channel is a tetramer, what is the fraction of open channels when 1, 2, 3, and 4 ligands are bound?
Question 13.22
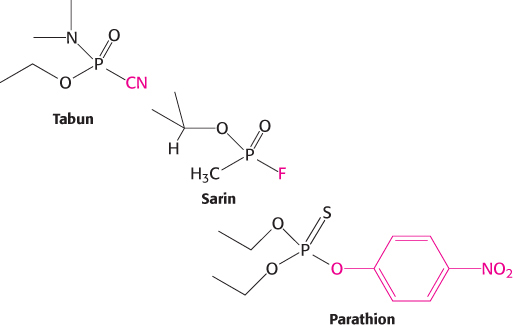
Respiratory paralysis. The neurotransmitter acetylcholine is degraded by a specific enzyme that is inactivated by Tabun, sarin, and parathion. On the basis of the structures below, propose a possible basis for their lethal actions.
395
Question 13.23
Ligand-
By what factor is the open-
to- closed ratio increased by the binding of the first acetylcholine molecule? The second acetylcholine molecule? What are the corresponding free-
energy contributions to channel opening at 25°C? Can the allosteric transition be accounted for by the MWC concerted model (Section 7.2)?
Question 13.24
Frog poison. Batrachotoxin (BTX) is a steroidal alkaloid from the skin of Phyllobates terribilis, a poisonous Colombian frog (the source of the poison used on blowgun darts). In the presence of BTX, Na+ channels in an excised patch stay persistently open when the membrane is depolarized. They close when the membrane is repolarized. Which transition is blocked by BTX?
Question 13.25
Valium target. γ-Aminobutyric acid (GABA) opens channels that are specific for chloride ions. The GABAA receptor channel is pharmacologically important because it is the target of Valium, which is used to diminish anxiety.
The extracellular concentration of Cl− is 123 mM and the intracellular concentration is 4 mM. In which direction does Cl− flow through an open channel when the membrane potential is in the −60 mV to +30 mV range?
What is the effect of Cl−-channel opening on the excitability of a neuron?
The hydropathy profile of the GABAA receptor resembles that of the acetylcholine receptor. Predict the number of subunits in this Cl− channel.
Question 13.26
Understanding SERCA. To study the mechanism of SERCA, you prepare membrane vesicles containing this protein oriented such that its ATP binding site is on the outer surface of the vesicle. To measure pump activity, you use an assay that detects the formation of inorganic phosphate in the medium. When you add calcium and ATP to the medium, you observe phosphate production for only a short period of time. Only after the addition of calcimycin, a molecule that makes membranes selectively permeable to calcium, do you observe sustained phosphate production. Explain.
Chapter Integration Problem
Question 13.27
Speed and efficiency matter. Acetylcholine is rapidly destroyed by the enzyme acetylcholinesterase. This enzyme, which has a turnover number of 25,000 per second, has attained catalytic perfection with a kcat/KM of 2 × 108 M−1s−1. Why is the efficiency of this enzyme physiologically crucial?
Mechanism Problem
Question 13.28
Remembrance of mechanisms past. Acetylcholinesterase converts acetylcholine into acetate and choline. Like serine proteases, acetylcholinesterase is inhibited by DIPF. Propose a catalytic mechanism for acetylcholine digestion by acetylcholinesterase. Show the reaction as chemical structures.
Data Interpretation Problems
Question 13.29
Tarantula toxin. Acid sensing is associated with pain, tasting, and other biological activities (Chapter 33). Acid sensing is carried out by a ligand -gated channel that permits Na+ influx in response to H+. This family of acid-
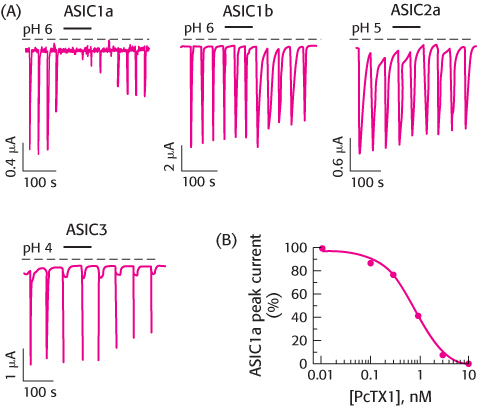
Which member of the ASIC family—
ASIC1a, ASIC1b, ASIC2a, or ASIC3— is most sensitive to the toxin? Is the effect of the toxin reversible? Explain.
What concentration of PcTX1 yields 50% inhibition of the sensitive channel?
Question 13.30
Channel problems 1. A number of pathological conditions result from mutations in the acetylcholine receptor channel. One such mutation in the β subunit, βV266M, causes muscle weakness and rapid fatigue. An investigation of the acetylcholine-
396
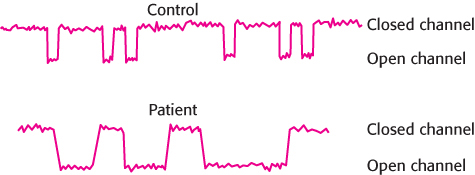
What is the effect of the mutation on channel function? Suggest some possible biochemical explanations for the effect.
Question 13.31
Channel problems 2. The acetylcholine receptor channel can also undergo mutation leading to fast-
Question 13.32
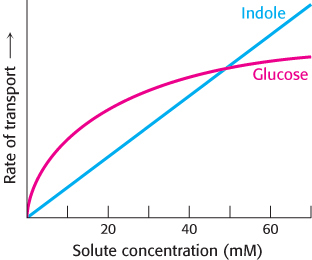
Transport differences. The rate of transport of two molecules, indole and glucose, across a cell membrane is shown below. What are the differences between the transport mechanisms of the two molecules? Suppose that ouabain inhibited the transport of glucose. What would this inhibition suggest about the mechanism of transport?