13.6 Specific Channels Increase the Permeability of Some Membranes to Water
One more important class of channels does not take part in ion transport at all. Instead, these channels increase the rate at which water flows through membranes. As noted in Section 12.3, membranes are reasonably permeable to water. Why, then, are water-specific channels required? In certain tissues, in some circumstances, rapid water transport through membranes is necessary. In the kidney, for example, water must be rapidly reabsorbed into the bloodstream after filtration. Similarly, in the secretion of saliva and tears, water must flow quickly through membranes. These observations suggested the existence of specific water channels, but initially the channels could not be identified.
The channels (now called aquaporins) were discovered serendipitously. Peter Agre noticed a protein present at high levels in red-blood-cell membranes that had been missed because the protein does not stain well with Coomassie blue. In addition to red blood cells, this protein was found in large quantities in tissues such as the kidney and the cornea, precisely the tissues thought to contain water channels. On the basis of this observation, further studies were designed, revealing that this 24-kDa membrane protein is, indeed, a water channel.
The structure of aquaporin has been determined (Figure 13.33). The protein consists of six membrane-spanning α helices. Two loops containing hydrophilic residues line the actual channel. Water molecules pass through in single file at a rate of 106 molecules per second. Importantly, specific positively charged residues toward the center of the channel prevent the transport of protons through aquaporin. Thus, aquaporin channels will not disrupt proton gradients, which play fundamental roles in energy transduction, as we will see in Chapter 18. Remarkably, the aquaporins are channels that have evolved specifically to conduct uncharged substrates.
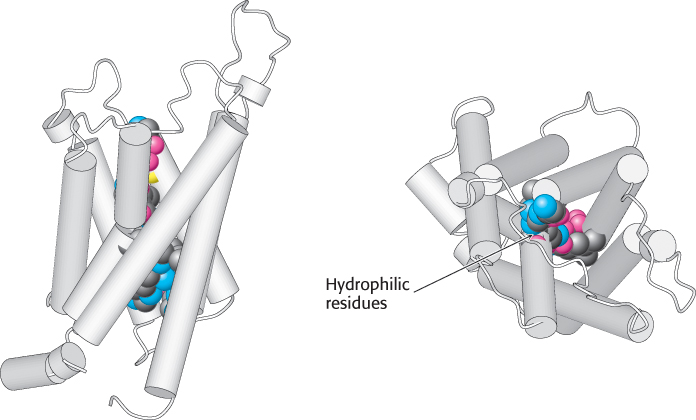
Figure 13.33:  Structure of aquaporin. The structure of aquaporin viewed from the side (left) and from the top (right). Notice the hydrophilic residues (shown as space-filling models) that line the water channel. [Drawn from 1J4N.pdb.]
Structure of aquaporin. The structure of aquaporin viewed from the side (left) and from the top (right). Notice the hydrophilic residues (shown as space-filling models) that line the water channel. [Drawn from 1J4N.pdb.]

 Structure of aquaporin. The structure of aquaporin viewed from the side (left) and from the top (right). Notice the hydrophilic residues (shown as space-
Structure of aquaporin. The structure of aquaporin viewed from the side (left) and from the top (right). Notice the hydrophilic residues (shown as space-