14.3 EGF Signaling: Signal-Transduction Pathways Are Poised to Respond
Our consideration of the signal-transduction cascades initiated by epinephrine and insulin included examples of how components of signal-transduction pathways are poised for action, ready to be activated by minor modifications. For example, G-protein subunits require only the binding of GTP in exchange for GDP to transmit a signal. This exchange reaction is thermodynamically favorable, but it is quite slow in the absence of an appropriate activated 7TM receptor. Similarly, the tyrosine kinase domains of the dimeric insulin receptor are ready for phosphorylation and activation but require insulin bound between two α subunits to draw the activation loop of one tyrosine kinase into the active site of a partner tyrosine kinase to initiate the signaling cascade.
Next, we examine a signal-transduction pathway that reveals another clear example of how these signaling cascades are poised to respond. This pathway is activated by the signal molecule epidermal growth factor (EGF). Like that of the insulin receptor, the initiator of this pathway is a receptor tyrosine kinase. Both the extracellular and the intracellular domains of this receptor are ready for action, held in check only by a specific structure that prevents receptors from coming together. Furthermore, in the EGF pathway, we will encounter several additional classes of signaling components that participate in many other signaling networks.
EGF binding results in the dimerization of the EGF receptor
Epidermal growth factor is a 6-kDa polypeptide that stimulates the growth of epidermal and epithelial cells (Figure 14.27). The EGF receptor (EGFR), like the insulin receptor, is a dimer of two identical subunits. Each subunit contains an intracellular protein tyrosine kinase domain that participates in cross-phosphorylation reactions (Figure 14.28). Unlike those of the insulin receptor, however, these units exist as monomers until they bind EGF. Moreover, each EGF receptor monomer binds a single molecule of EGF in its extracellular domain (Figure 14.29). Thus the dimer binds two ligand molecules, in contrast with the insulin-receptor dimer, which binds only one ligand. Note that each EGF molecule lies far away from the dimer interface. This interface includes a so-called dimerization arm from each monomer that reaches out and inserts into a binding pocket on the other monomer.
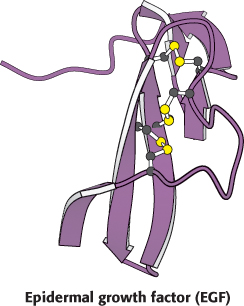
Figure 14.27:  Structure of epidermal growth factor. Notice that three intrachain disulfide bonds stabilize the compact three-dimensional structure of the growth factor.
Structure of epidermal growth factor. Notice that three intrachain disulfide bonds stabilize the compact three-dimensional structure of the growth factor.
[Drawn from 1EGF.pdb.]

Figure 14.28: Modular structure of the EGF receptor. This schematic view of the amino acid sequence of the EGF receptor shows the EGF-binding domain that lies outside the cell, a single transmembrane helix-forming region, the intracellular tyrosine kinase domain, and the tyrosine-rich domain at the carboxyl terminus.
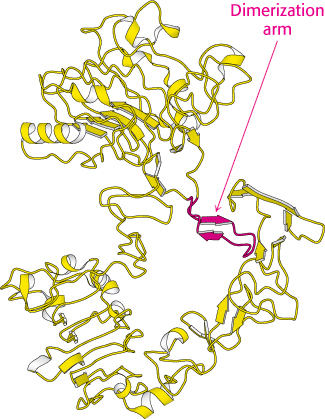
Figure 14.30:  Structure of the unactivated EGF receptor. The extracellular domain of the EGF receptor is shown in the absence of bound EGF. Notice that the dimerization arm is bound to a part of the receptor that makes it unavailable for interaction with the other receptor.
Structure of the unactivated EGF receptor. The extracellular domain of the EGF receptor is shown in the absence of bound EGF. Notice that the dimerization arm is bound to a part of the receptor that makes it unavailable for interaction with the other receptor.
[Drawn from 1NQL.pdb.]
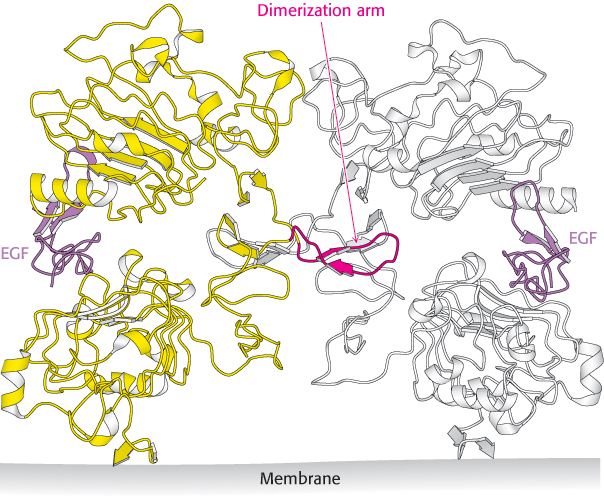
Figure 14.29: EGF receptor dimerization. The structure of the extracellular region of the EGF receptor is shown bound to EGF. Notice that the structure is dimeric with one EGF molecule bound to each receptor molecule and that the dimerization is mediated by a dimerization arm that extends from each receptor molecule.
[Drawn from 1IVO.pdb.]
Although this structure nicely reveals the interactions that support the formation of a receptor dimer favoring cross-phosphorylation, it raises another question: Why doesn’t the receptor dimerize and signal in the absence of EGF? This question has been addressed by examining the structure of the EGF receptor in the absence of bound ligand (Figure 14.30). This structure is, indeed, monomeric, and each monomer is in a conformation that is quite different from that observed in the ligand-bound dimer. In particular, the dimerization arm binds to a domain within the same monomer that holds the receptor in a closed configuration. In essence, the receptor is poised in a spring-loaded conformation held in position by the contact between the interaction loop and another part of the structure, ready to bind ligand and change into a conformation active for dimerization and signaling.
This observation suggests that a receptor that exists in the extended conformation even in the absence of bound ligand would be constitutively active. Remarkably, such a receptor exists. This receptor, HER2, is approximately 50% identical in amino acid sequence with the EGF receptor and has the same domain structure. HER2 does not bind any known ligand, yet crystallographic studies reveal that it adopts an extended structure very similar to that observed for the ligand-bound EGF receptor. Under normal conditions, HER2 forms heterodimers with the EGF receptor and other members of the EGF receptor family and participates in cross-phosphorylation reactions with these receptors. HER2 is overexpressed in some cancers, presumably contributing to tumor growth by forming homodimers that signal even in the absence of ligand. We will return to HER2 when we consider approaches to cancer treatment based on knowledge of signaling pathways (Section 14.5).
The EGF receptor undergoes phosphorylation of its carboxyl-terminal tail
Like the insulin receptor, the EGF receptor undergoes cross-phosphorylation of one unit by another unit within a dimer. However, unlike that of the insulin receptor, the site of this phosphorylation is not within the activation loop of the kinase, but rather in a region that lies on the C-terminal side of the kinase domain. As many as five tyrosine residues in this region are phosphorylated. The dimerization of the EGF receptor brings the C-terminal region on one receptor into the active site of its partner’s kinase. The kinase itself is in an active conformation without phosphorylation, revealing again how this signaling system is poised to respond.
EGF signaling leads to the activation of Ras, a small G protein
The phosphotyrosines on the EGF receptors act as docking sites for SH2 domains on other proteins. The intracellular signaling cascade begins with the binding of Grb2, a key adaptor protein that contains one SH2 domain and two Src homology 3 (SH3) domains. On phosphorylation of the receptor, the SH2 domain of Grb2 binds to the phosphotyrosine residues of the receptor tyrosine kinase. Through its two SH3 domains, Grb2 then binds polyproline-rich polypeptides within a protein called Sos. Sos, in turn, binds to Ras and activates it. A very prominent signal- transduction component, Ras is a member of a class of proteins called the small G proteins. Like the G proteins described in Section 14.1, the small G proteins contain bound GDP in their unactivated forms. Sos opens up the nucleotide-binding pocket of Ras, allowing GDP to escape and GTP to enter in its place. Because of its effect on Ras, Sos is referred to as a guanine-nucleotide-exchange factor (GEF). Thus, the binding of EGF to its receptor leads to the conversion of Ras into its GTP form through the intermediacy of Grb2 and Sos (Figure 14.31).
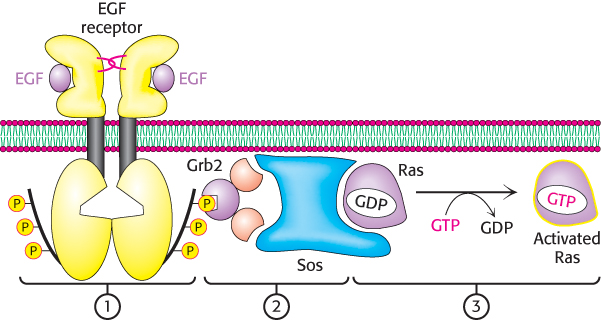
Figure 14.31: Ras activation mechanism. The dimerization of the EGF receptor due to EGF binding leads to: (1) the phosphorylation of the C-terminal tails of the receptor, (2) the subsequent recruitment of Grb2 and Sos, and (3) the exchange of GTP for GDP in Ras. This signal-transduction pathway results in the conversion of Ras into its activated GTP-bound form.
Activated Ras initiates a protein kinase cascade
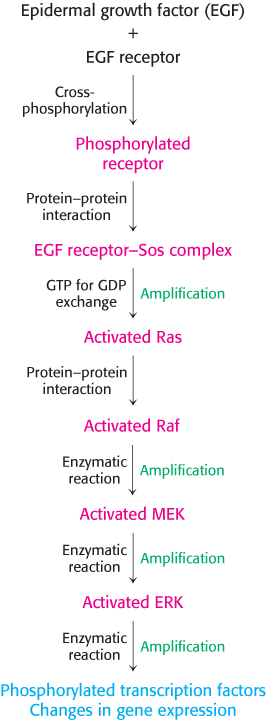
Figure 14.32: EGF signaling pathway. The key steps in the pathway initiated by EGF binding to the EGF receptor. A kinase cascade leads to the phosphorylation of transcription factors and concomitant changes in gene expression.
Ras changes conformation when it is transformed from its GDP into its GTP form. In the GTP form, Ras binds other proteins, including a protein kinase termed Raf. When bound to Ras, Raf undergoes a conformational change that activates the Raf protein kinase domain. Both Ras and Raf are anchored to the membrane through covalently bound lipid modifications. Activated Raf then phosphorylates other proteins, including protein kinases termed MEKs. In turn, MEKs activate kinases called extracellular signal-regulated kinases (ERKs). ERKs then phosphorylate numerous substrates, including transcription factors in the nucleus as well as other protein kinases. The complete flow of information from the arrival of EGF at the cell surface to changes in gene expression is summarized in Figure 14.32.
 Small G proteins, or small GTPases, constitute a large superfamily of proteins—grouped into subfamilies called Ras, Rho, Arf, Rab, and Ran—that play a major role in a host of cell functions including growth, differentiation, cell motility, cytokinesis (the separation of two cells during division), and the transport of materials throughout the cell (Table 14.2). As with the heterotrimeric G proteins, the small G proteins cycle between an active GTP-bound form and an inactive GDP-bound form. They differ from the heterotrimeric G proteins in being smaller (20–25 kDa versus 30–35 kDa) and monomeric. Nonetheless, the two families are related by divergent evolution, and small G proteins have many key mechanistic and structural motifs in common with the Gα subunit of the heterotrimeric G proteins.
Small G proteins, or small GTPases, constitute a large superfamily of proteins—grouped into subfamilies called Ras, Rho, Arf, Rab, and Ran—that play a major role in a host of cell functions including growth, differentiation, cell motility, cytokinesis (the separation of two cells during division), and the transport of materials throughout the cell (Table 14.2). As with the heterotrimeric G proteins, the small G proteins cycle between an active GTP-bound form and an inactive GDP-bound form. They differ from the heterotrimeric G proteins in being smaller (20–25 kDa versus 30–35 kDa) and monomeric. Nonetheless, the two families are related by divergent evolution, and small G proteins have many key mechanistic and structural motifs in common with the Gα subunit of the heterotrimeric G proteins.
|
|
|
|
|
Regulates cell growth through serine–threonine protein kinases |
|
|
Reorganizes cytoskeleton through serine–threonine protein kinases |
|
|
Activates the ADP-ribosyltransferase of the cholera toxin A subunit; regulates vesicular trafficking pathways; activates phospholipase D |
|
|
Plays a key role in secretory and endocytotic pathways |
|
|
Functions in the transport of RNA and protein into and out of the nucleus |
Table 14.2: Ras superfamily of GTPases
EGF signaling is terminated by protein phosphatases and the intrinsic GTPase activity of Ras
Because so many components of the EGF signal-transduction pathway are activated by phosphorylation, we can expect protein phosphatases to play key roles in the termination of EGF signaling. Indeed, crucial phosphatases remove phosphoryl groups from tyrosine residues on the EGF receptor and from serine, threonine, and tyrosine residues in the protein kinases that participate in the signaling cascade. The signaling process itself sets in motion the events that activate many of these phosphatases. Consequently, signal activation also initiates signal termination.
Like the G proteins activated by 7TM receptors, Ras possesses intrinsic GTPase activity. Thus, the activated GTP form of Ras spontaneously converts into the inactive GDP form. The rate of conversion can be accelerated in the presence of GTPase-activating proteins (GAPs), which interact with small G proteins in the GTP form and facilitate GTP hydrolysis. Thus, the lifetime of activated Ras is regulated by accessory proteins in the cell. The GTPase activity of Ras is crucial for shutting off signals leading to cell growth, and so it is not surprising that mutations in Ras are found in many types of cancer, as discussed in Section 14.5.

 Structure of epidermal growth factor. Notice that three intrachain disulfide bonds stabilize the compact three-
Structure of epidermal growth factor. Notice that three intrachain disulfide bonds stabilize the compact three-

 Structure of the unactivated EGF receptor. The extracellular domain of the EGF receptor is shown in the absence of bound EGF. Notice that the dimerization arm is bound to a part of the receptor that makes it unavailable for interaction with the other receptor.
Structure of the unactivated EGF receptor. The extracellular domain of the EGF receptor is shown in the absence of bound EGF. Notice that the dimerization arm is bound to a part of the receptor that makes it unavailable for interaction with the other receptor.



 Small G proteins, or small GTPases, constitute a large superfamily of proteins—
Small G proteins, or small GTPases, constitute a large superfamily of proteins—