15.4 Metabolic Pathways Contain Many Recurring Motifs
At first glance, metabolism appears intimidating because of the sheer number of reactants and reactions. Nevertheless, there are unifying themes that make the comprehension of this complexity more manageable. These unifying themes include common metabolites, reactions, and regulatory schemes that stem from a common evolutionary heritage.
Activated carriers exemplify the modular design and economy of metabolism
We have seen that phosphoryl transfer can be used to drive otherwise endergonic reactions, alter the energy of conformation of a protein, or serve as a signal to alter the activity of a protein. The phosphoryl-
1. Activated Carriers of Electrons for Fuel Oxidation. In aerobic organisms, the ultimate electron acceptor in the oxidation of fuel molecules is O2. However, electrons are not transferred directly to O2. Instead, fuel molecules transfer electrons to special carriers, which are either pyridine nucleotides or flavins. The reduced forms of these carriers then transfer their high-
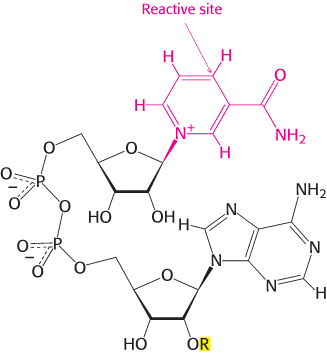
Nicotinamide adenine dinucleotide is a major electron carrier in the oxidation of fuel molecules (Figure 15.13). The reactive part of NAD+ is its nicotinamide ring, a pyridine derivative synthesized from the vitamin niacin. In the oxidation of a substrate, the nicotinamide ring of NAD+ accepts a hydrogen ion and two electrons, which are equivalent to a hydride ion (H:−). The reduced form of this carrier is called NADH. In the oxidized form, the nitrogen atom carries a positive charge, as indicated by NAD+. NAD+ is the electron acceptor in many reactions of the type
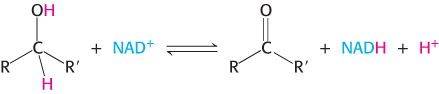
 .
.
In this dehydrogenation, one hydrogen atom of the substrate is directly transferred to NAD+, whereas the other appears in the solvent as a proton. Both electrons lost by the substrate are transferred to the nicotinamide ring.
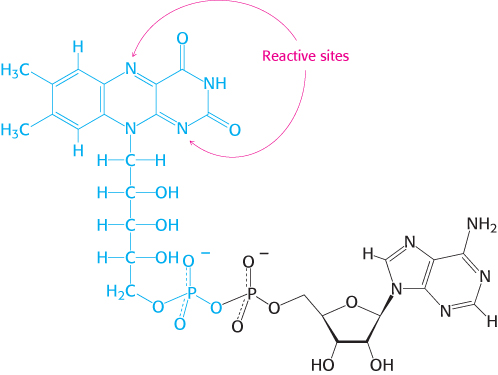
The other major electron carrier in the oxidation of fuel molecules is the coenzyme flavin adenine dinucleotide (Figure 15.14). The abbreviations for the oxidized and reduced forms of this carrier are FAD and FADH2, respectively. FAD is the electron acceptor in reactions of the type
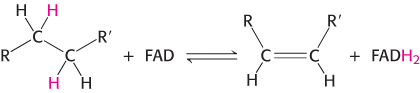
436
The reactive part of FAD is its isoalloxazine ring, a derivative of the vitamin riboflavin (Figure 15.15). FAD, like NAD+, can accept two electrons. In doing so, FAD, unlike NAD+, takes up two protons. These carriers of high-
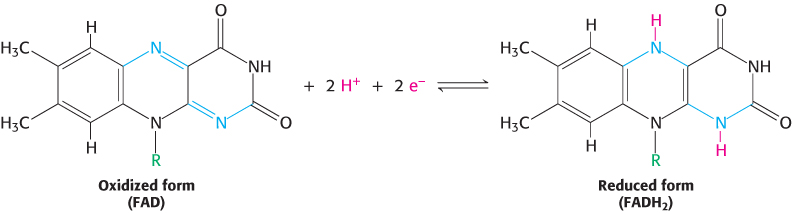
2. An Activated Carrier of Electrons for Reductive Biosynthesis. High-
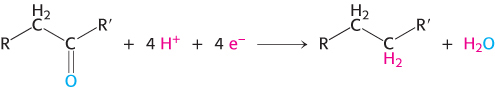
The electron donor in most reductive biosyntheses is NADPH, the reduced form of nicotinamide adenine dinucleotide phosphate (NADP+; Figure 15.13). NADPH differs from NADH in that the 2′ -hydroxyl group of its adenosine moiety is esterified with phosphate. NADPH carries electrons in the same way as NADH. However, NADPH is used almost exclusively for reductive biosyntheses, whereas NADH is used primarily for the generation of ATP. The extra phosphoryl group on NADPH is a tag that enables enzymes to distinguish between high-
437
3. An Activated Carrier of Two-
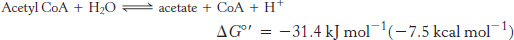
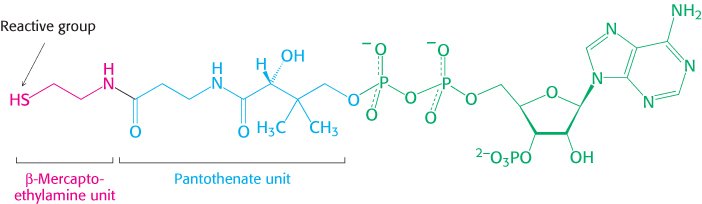
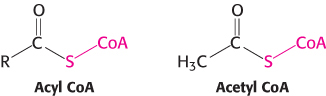
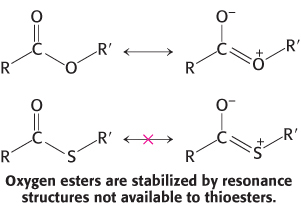
A thioester is thermodynamically more unstable than an oxygen ester because the electrons of the C O bond cannot form resonance structures with the C
O bond cannot form resonance structures with the C S bond that are as stable as those that they can form with the C
S bond that are as stable as those that they can form with the C O bond. Consequently, acetyl CoA has a high acetyl-
O bond. Consequently, acetyl CoA has a high acetyl-
The use of activated carriers illustrates two key aspects of metabolism. First, NADH, NADPH, and FADH2 react slowly with O2 in the absence of a catalyst. Likewise, ATP and acetyl CoA are hydrolyzed slowly (over many hours or even days) in the absence of a catalyst. These molecules are kinetically quite stable in the face of a large thermodynamic driving force for reaction with O2 (in regard to the electron carriers) and H2O (for ATP and acetyl CoA). The kinetic stability of these molecules in the absence of specific catalysts is essential for their biological function because it enables enzymes to control the flow of free energy and reducing power.
Second, most interchanges of activated groups in metabolism are accomplished by a rather small set of carriers (Table 15.2). The existence of a recurring set of activated carriers in all organisms is one of the unifying motifs of biochemistry. Furthermore, it illustrates the modular design of metabolism. A small set of molecules carries out a very wide range of tasks. Metabolism is readily comprehended because of the economy and elegance of its underlying design.
|
Carrier molecule in activated form |
Group carried |
Vitamin precursor |
|---|---|---|
|
ATP |
Phosphoryl |
|
|
NADH and NADPH |
Electrons |
Nicotinate (niacin) (vitamin B3) |
|
FADH2 |
Electrons |
Riboflavin (vitamin B2) |
|
FMNH2 |
Electrons |
Riboflavin (vitamin B2) |
|
Coenzyme A |
Acyl |
Pantothenate (vitamin B5) |
|
Lipoamide |
Acyl |
|
|
Thiamine pyrophosphate |
Aldehyde |
Thiamine (vitamin B1) |
|
Biotin |
CO2 |
Biotin (vitamin B7) |
|
Tetrahydrofolate |
One- |
Folate (vitamin B9) |
|
S-Adenosylmethionine |
Methyl |
|
|
Uridine diphosphate glucose |
Glucose |
|
|
Cytidine diphosphate diacylglycerol |
Phosphatidate |
|
|
Nucleoside triphosphates |
Nucleotides |
|
|
Note: Many of the activated carriers are coenzymes that are derived from water- |
||
438
Many activated carriers are derived from vitamins
Almost all the activated carriers that act as coenzymes are derived from vitamins. Vitamins are organic molecules that are needed in small amounts in the diets of some higher animals. Table 15.3 lists the vitamins that act as coenzymes and Figure 15.17 shows the structures of some of them. This series of vitamins is known as the vitamin B group. In all cases, the vitamin must be modified before it can serve its function. We have already touched on the roles of niacin, riboflavin, and pantothenate. We will see these three and the other B vitamins many times in our study of biochemistry.
|
Vitamin |
Coenzyme |
Typical reaction type |
Consequences of deficiency |
|---|---|---|---|
|
Thiamine (B1) |
Thiamine pyrophosphate |
Aldehyde transfer |
Beriberi (weight loss, heart problems, neurological dysfunction) |
|
Riboflavin (B2) |
Flavin adenine dinucleotide (FAD) |
Oxidation– |
Cheliosis and angular stomatitis (lesions of the mouth), dermatitis |
|
Pyridoxine (B6) |
Pyridoxal phosphate |
Group transfer to or from amino acids |
Depression, confusion, convulsions |
|
Nicotinic acid (niacin) (B3) |
Nicotinamide adenine dinucleotide (NAD+) |
Oxidation– |
Pellagra (dermatitis, depression, diarrhea) |
|
Pantothenic acid (B5) |
Coenzyme A |
Acyl- |
Hypertension |
|
Biotin (B7) |
Biotin– |
ATP- |
Rash about the eyebrows, muscle pain, fatigue (rare) |
|
Folic acid (B9) |
Tetrahydrofolate |
Transfer of one- |
Anemia, neural- |
|
B12 |
5′-Deoxyadenosyl cobalamin |
Transfer of methyl groups; intramolecular rearrangements |
Anemia, pernicious anemia, methylmalonic acidosis |
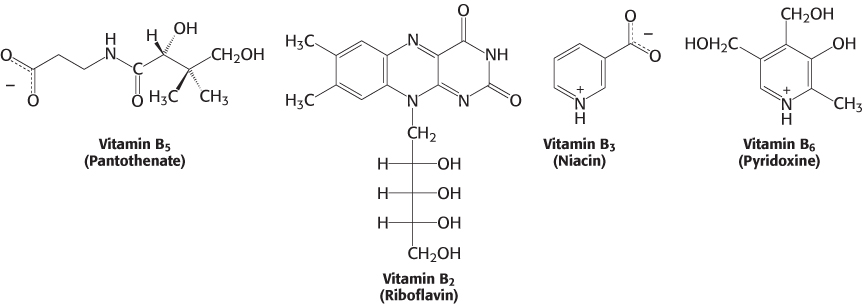
 Vitamins serve the same roles in nearly all forms of life, but higher animals lost the capacity to synthesize them in the course of evolution. For instance, whereas E. coli can thrive on glucose and organic salts, human beings require at least 12 vitamins in their diet. The biosynthetic pathways for vitamins can be complex; thus, it is biologically more efficient to ingest vitamins than to synthesize the enzymes required to construct them from simple molecules. This efficiency comes at the cost of dependence on other organisms for chemicals essential for life. Indeed, vitamin deficiency can generate diseases in all organisms requiring these molecules (Tables 15.3 and 15.4).
Vitamins serve the same roles in nearly all forms of life, but higher animals lost the capacity to synthesize them in the course of evolution. For instance, whereas E. coli can thrive on glucose and organic salts, human beings require at least 12 vitamins in their diet. The biosynthetic pathways for vitamins can be complex; thus, it is biologically more efficient to ingest vitamins than to synthesize the enzymes required to construct them from simple molecules. This efficiency comes at the cost of dependence on other organisms for chemicals essential for life. Indeed, vitamin deficiency can generate diseases in all organisms requiring these molecules (Tables 15.3 and 15.4).
|
Vitamin |
Function |
Deficiency |
|---|---|---|
|
A |
Roles in vision, growth, reproduction |
Night blindness, cornea damage, damage to respiratory and gastrointestinal tract |
|
C (ascorbic acid) |
Antioxidant |
Scurvy (swollen and bleeding gums, subdermal hemorrhaging) |
|
D |
Regulation of calcium and phosphate metabolism |
Rickets (children): skeletal deformities, impaired growth Osteomalacia (adults): soft, bending bones |
|
E |
Antioxidant |
Lesions in muscles and nerves (rare) |
|
K |
Blood coagulation |
Subdermal hemorrhaging |
439
Not all vitamins function as coenzymes. Vitamins designated by the letters A, C, D, E, and K (Figure 15.18 and Table 15.4) have a diverse array of functions. Vitamin A (retinol) is the precursor of retinal, the light-
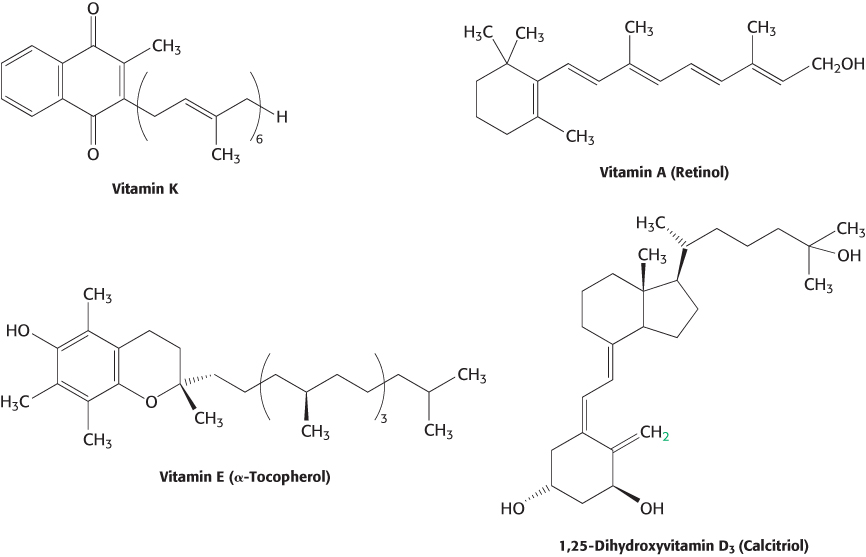
440
Key reactions are reiterated throughout metabolism
Just as there is an economy of design in the use of activated carriers, so is there an economy of design in biochemical reactions. The thousands of metabolic reactions, bewildering at first in their variety, can be subdivided into just six types (Table 15.5). Specific reactions of each type appear repeatedly, reducing the number of reactions that a student needs to learn.
|
Type of reaction |
Description |
|---|---|
|
Oxidation– |
Electron transfer |
|
Ligation requiring ATP cleavage |
Formation of covalent bonds (i.e., carbon– |
|
Isomerization |
Rearrangement of atoms to form isomers |
|
Group transfer |
Transfer of a functional group from one molecule to another |
|
Hydrolytic |
Cleavage of bonds by the addition of water |
|
Carbon bond cleavage by means other than hydrolysis or oxidation |
Two substrates yielding one product or vice versa. When H2O or CO2 are a product, a double bond is formed. |
1. Oxidation–
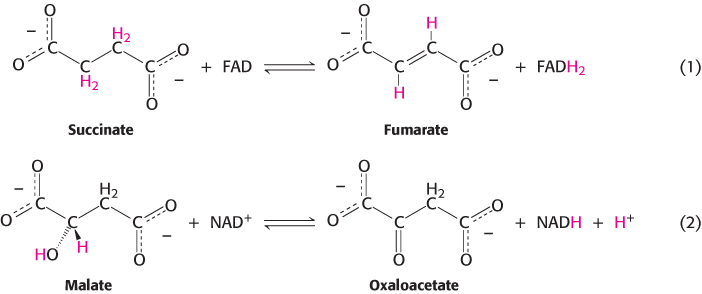
These two oxidation–
2. Ligation reactions form bonds by using free energy from ATP cleavage. Reaction 3 illustrates the ATP-
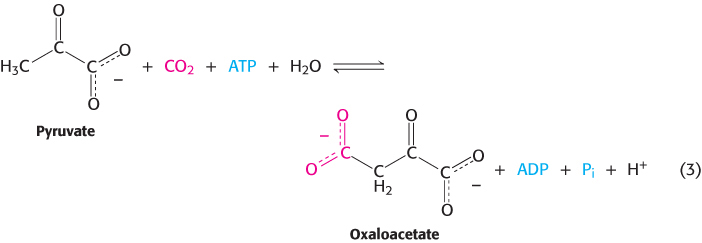
441
The oxaloacetate can be used in the citric acid cycle, or converted into glucose or amino acids such as aspartic acid.
3. Isomerization reactions rearrange particular atoms within a molecule. Their role is often to prepare the molecule for subsequent reactions such as the oxidation–
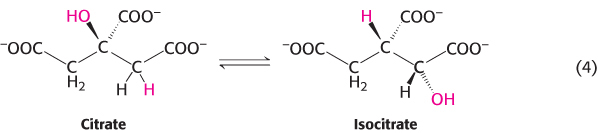
Reaction 4 is, again, a component of the citric acid cycle. This isomerization prepares the molecule for subsequent oxidation and decarboxylation reactions by moving the hydroxyl group of citrate from a tertiary to a secondary position.
4. Group-
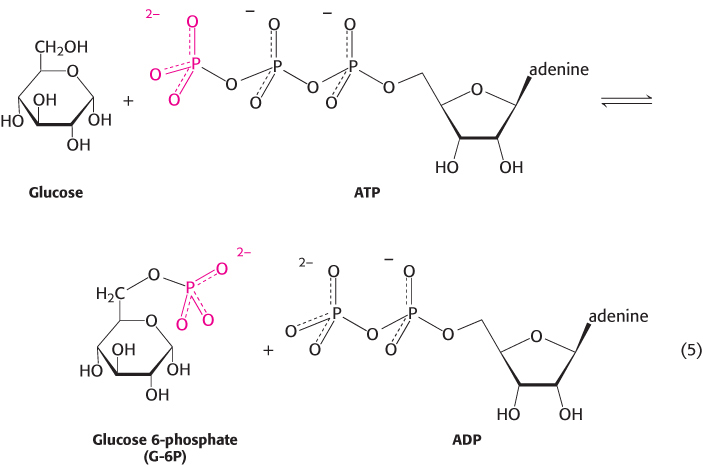
As stated earlier, group-
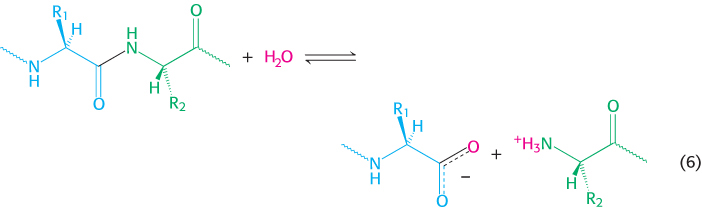
5. Hydrolytic reactions cleave bonds by the addition of water. Hydrolysis is a common means of degrading large molecules, either to facilitate further metabolism or to reuse some of the components for biosynthetic purposes. Proteins are digested by hydrolytic cleavage (chapters 9 and 10). Reaction 6 illustrates the hydrolysis of a peptide to yield two smaller peptides.
442
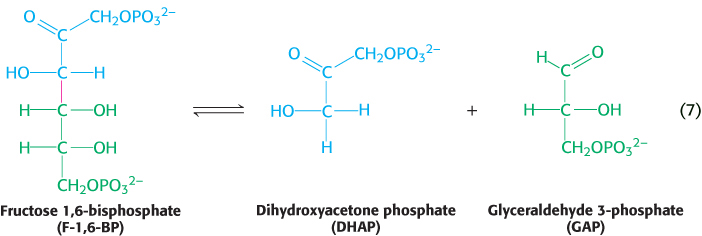
6. Carbon bonds can be cleaved by means other than hydrolysis or oxidation, with two substrates yielding one product or vice versa. When CO2 or H2O is released, a double bond is formed. The enzymes that catalyze these types of reaction are classified as lyases. An important example, illustrated in reaction 7, is the conversion of the six-
This reaction is a critical step in glycolysis (Chapter 16). Dehydrations to form double bonds, such as the formation of phosphoenolpyruvate (Figure 15.6) from 2-
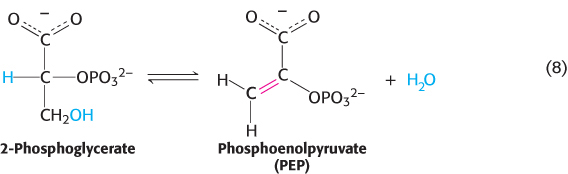
The dehydration sets up the next step in the pathway, a group-
These six fundamental reaction types are the basis of metabolism. Remember that all six types can proceed in either direction, depending on the standard free energy for the specific reaction and the concentrations of the reactants and products inside the cell. An effective way to learn is to look for commonalities in the diverse metabolic pathways that we will be examining. There is a chemical logic that, when exposed, renders the complexity of the chemistry of living systems more manageable and reveals its elegance.
Metabolic processes are regulated in three principal ways
It is evident that the complex network of metabolic reactions must be rigorously regulated. The levels of available nutrients must be monitored and the activity of metabolic pathways must be altered and integrated to create homeostasis, a stable biochemical environment. At the same time, metabolic control must be flexible, able to adjust metabolic activity to the constantly changing external environments of cells. Figure 15.19 illustrates the nutrient pools and their connections that must be monitored and regulated. Metabolism is regulated through control of (1) the amounts of enzymes, (2) their catalytic activities, and (3) the accessibility of substrates.
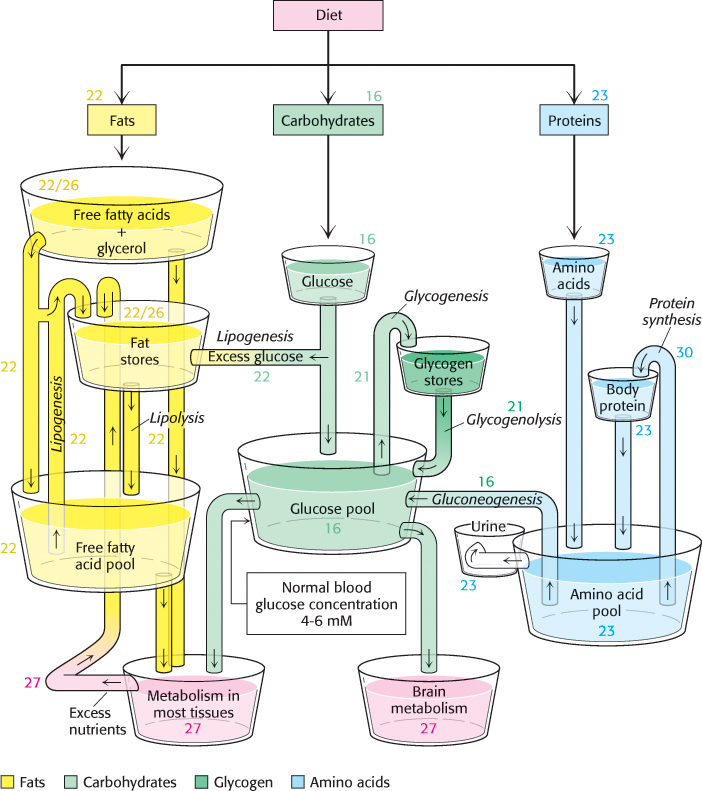
443
Controlling the amounts of enzymes. The amount of a particular enzyme depends on both its rate of synthesis and its rate of degradation. The level of many enzymes is adjusted by a change in the rate of transcription of the genes encoding them (chapters 29 and 31). In E. coli, for example, the presence of lactose induces within minutes a more than 50-
Controlling catalytic activity. The catalytic activity of enzymes is controlled in several ways. Allosteric control is especially important. For example, the first reaction in many biosynthetic pathways is allosterically inhibited by the ultimate product of the pathway. The inhibition of aspartate transcarbamoylase by cytidine triphosphate (Section 10.1) is a well-
Hormones coordinate metabolic relations between different tissues, often by regulating the reversible modification of key enzymes. For instance, the hormone epinephrine triggers a signal-
444
Many reactions in metabolism are controlled by the energy status of the cell. One index of the energy status is the energy charge, which is proportional to the mole fraction of ATP plus half the mole fraction of ADP, given that ATP contains two anhydride bonds, whereas ADP contains one. Hence, the energy charge is defined as
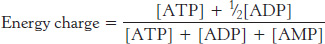
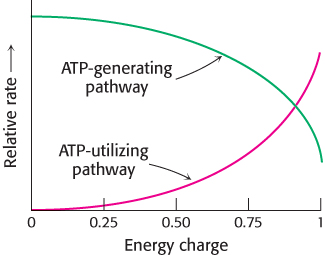
The energy charge can have a value ranging from 0 (all AMP) to 1 (all ATP). ATP-

The phosphorylation potential, in contrast with the energy charge, depends on the concentration of Pi and is directly related to the free-
Controlling the accessibility of substrates. Controlling the availability of substrates is another means of regulating metabolism in all organisms. For instance, glucose breakdown can take place in many cells only if insulin is present to promote the entry of glucose into the cell. In eukaryotes, metabolic regulation and flexibility are enhanced by compartmentalization. The transfer of substrates from one compartment of a cell to another can serve as a control point. For example, fatty acid oxidation takes place in mitochondria, where as fatty acid synthesis takes place in the cytoplasm. Compartmentalization segregates opposed reactions.
Aspects of metabolism may have evolved from an RNA world
 How did the complex pathways that constitute metabolism evolve? The current thinking is that RNA was an early biomolecule that dominated metabolism, serving both as a catalyst and an information storage molecule. This hypothetical time is called the RNA world.
How did the complex pathways that constitute metabolism evolve? The current thinking is that RNA was an early biomolecule that dominated metabolism, serving both as a catalyst and an information storage molecule. This hypothetical time is called the RNA world.
Why do activated carriers such as ATP, NADH, FADH2, and coenzyme A contain adenosine diphosphate units? A possible explanation is that these molecules evolved from the early RNA catalysts. Non-
445