16.2 The Glycolytic Pathway Is Tightly Controlled
The glycolytic pathway has a dual role: it degrades glucose to generate ATP and it provides building blocks for biosynthetic reactions. The rate of conversion of glucose into pyruvate is regulated to meet these two major cellular needs. In metabolic pathways, enzymes catalyzing essentially irreversible reactions are potential sites of control. In glycolysis, the reactions catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase are virtually irreversible, and each of them serves as a control site. These enzymes become more active or less so in response to the reversible binding of allosteric effectors or to covalent modification. In addition, the amounts of these important enzymes are varied by the regulation of transcription to meet changing metabolic needs. The time required for allosteric control, regulation by phosphorylation, and transcriptional control is measured typically in milliseconds, seconds, and hours, respectively. We will consider the control of glycolysis in two different tissues—skeletal muscle and liver.
Phosphofructokinase.
Phosphofructokinase is the most important control site in the mammalian glycolytic pathway (Figure 16.16). High levels of ATP allosterically inhibit the enzyme (a 340-kDa tetramer). ATP binds to a specific regulatory site that is distinct from the catalytic site. The binding of ATP lowers the enzyme’s affinity for fructose 6-phosphate. Thus, a high concentration of ATP converts the hyperbolic binding curve of fructose 6-phosphate into a sigmoidal one (Figure 16.17). AMP reverses the inhibitory action of ATP, and so the activity of the enzyme increases when the ATP/AMP ratio is lowered. In other words, glycolysis is stimulated as the energy charge falls. A decrease in pH also inhibits phosphofructokinase activity by augmenting the inhibitory effect of ATP. The pH might fall when fast twitch muscle is functioning anaerobically, producing excessive quantities of lactic acid. The inhibitory effect protects the muscle from damage that would result from the accumulation of too much acid.
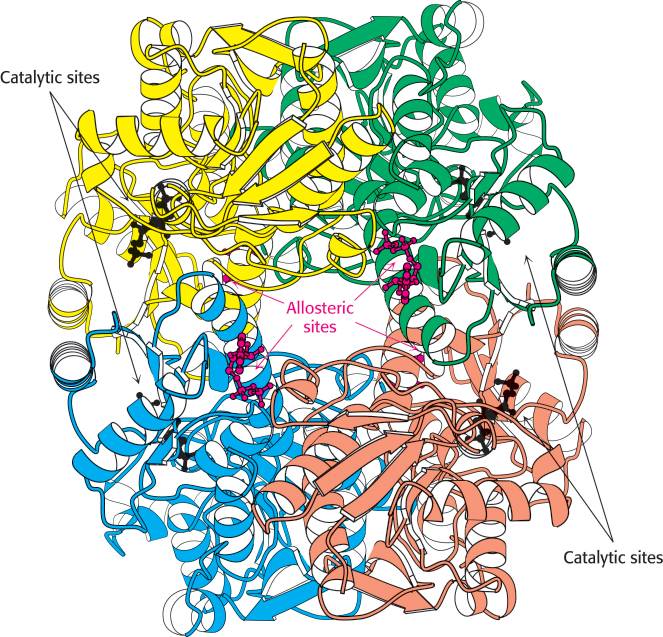
Figure 16.16:  Structure of phosphofructokinase. The structure of phosphofructokinase from E. coli comprises a tetramer of four identical subunits. Notice the separation of the catalytic and allosteric sites. Each subunit of the human liver enzyme consists of two domains that are similar to the E. coli enzyme.
Structure of phosphofructokinase. The structure of phosphofructokinase from E. coli comprises a tetramer of four identical subunits. Notice the separation of the catalytic and allosteric sites. Each subunit of the human liver enzyme consists of two domains that are similar to the E. coli enzyme.
[Drawn from 1PFK.pdb.]
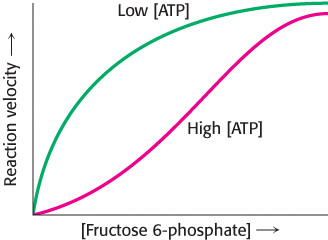
Figure 16.17: Allosteric regulation of phosphofructokinase. A high level of ATP inhibits the enzyme by decreasing its affinity for fructose 6-phosphate.
Why is AMP and not ADP the positive regulator of phosphofructokinase? When ATP is being utilized rapidly, the enzyme adenylate kinase (Section 9.4) can form ATP from ADP by the following reaction:
Thus, some ATP is salvaged from ADP, and AMP becomes the signal for the low-energy state. Moreover, the use of AMP as an allosteric regulator provides an especially sensitive control. We can understand why by considering, first, that the total adenylate pool ([ATP], [ADP], [AMP]) in a cell is constant over the short term and, second, that the concentration of ATP is greater than that of ADP and the concentration of ADP is, in turn, greater than that of AMP. Consequently, small-percentage changes in [ATP] result in larger-percentage changes in the concentrations of the other adenylate nucleotides. This magnification of small changes in [ATP] to larger changes in [AMP] leads to tighter control by increasing the range of sensitivity of phosphofructokinase (Problem 46).
Hexokinase.
Phosphofructokinase is the most prominent regulatory enzyme in glycolysis, but it is not the only one. Hexokinase, the enzyme catalyzing the first step of glycolysis, is inhibited by its product, glucose 6-phosphate. High concentrations of this molecule signal that the cell no longer requires glucose for energy or for the synthesis of glycogen, a storage form of glucose (Chapter 21), and the glucose will be left in the blood. A rise in glucose 6-phosphate concentration is a means by which phosphofructokinase communicates with hexokinase. When phosphofructokinase is inactive, the concentration of fructose 6-phosphate rises. In turn, the level of glucose 6-phosphate rises because it is in equilibrium with fructose 6-phosphate. Hence, the inhibition of phosphofructokinase leads to the inhibition of hexokinase.
Why is phosphofructokinase rather than hexokinase the pacemaker of glycolysis? The reason becomes evident on noting that glucose 6-phosphate is not solely a glycolytic intermediate. In muscle, glucose 6-phosphate can also be converted into glycogen. The first irreversible reaction unique to the glycolytic pathway, the committed step (Section 10.1), is the phosphorylation of fructose 6-phosphate to fructose 1,6-bisphosphate. Thus, it is highly appropriate for phosphofructokinase to be the primary control site in glycolysis. In general, the enzyme catalyzing the committed step in a metabolic sequence is the most important control element in the pathway.
Pyruvate kinase.
Pyruvate kinase, the enzyme catalyzing the third irreversible step in glycolysis, controls the outflow from this pathway. This final step yields ATP and pyruvate, a central metabolic intermediate that can be oxidized further or used as a building block. ATP allosterically inhibits pyruvate kinase to slow glycolysis when the energy charge is high. When the pace of glycolysis increases, fructose 1,6-bisphosphate, the product of the preceding irreversible step in glycolysis, activates the kinase to enable it to keep pace with the oncoming high flux of intermediates. A summary of the regulation of glycolysis in resting and active muscle is shown in Figure 16.18.
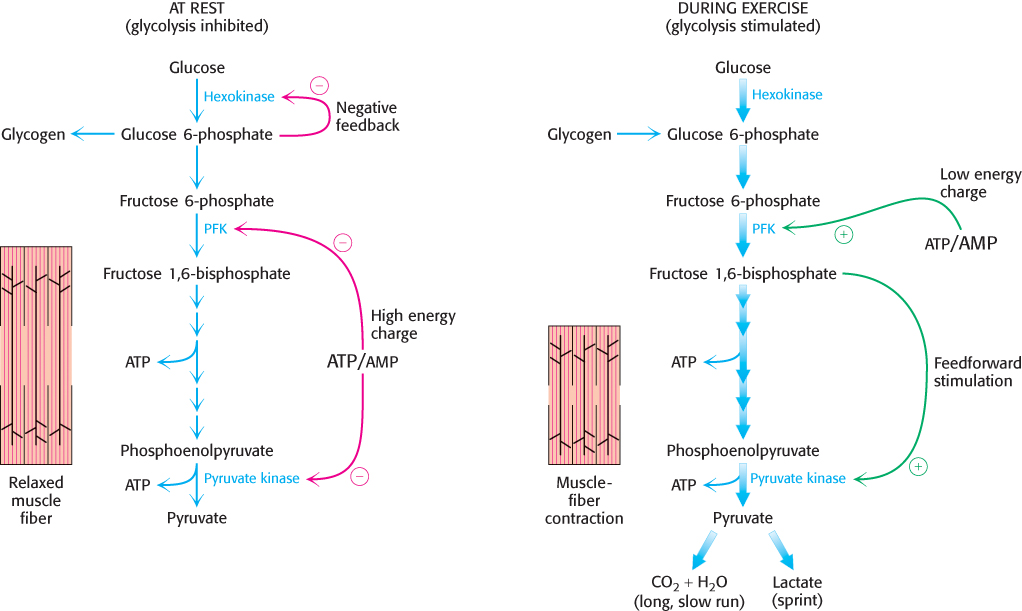
Figure 16.18: Regulation of glycolysis in muscle. At rest (left), glycolysis is not very active (thin arrows). The high concentration of ATP inhibits phosphofructokinase (PFK), pyruvate kinase, and hexokinase. Glucose 6-phosphate is converted into glycogen (Chapter 21). During exercise (right), the decrease in the ATP/AMP ratio resulting from muscle contraction activates phosphofructokinase and hence glycolysis. The flux down the pathway is increased, as represented by the thick arrows.
Phosphofructokinase.
Liver phosphofructokinase can be regulated by ATP as in muscle, but such regulation is not as important since the liver does not experience the sudden ATP needs that a contracting muscle does. Likewise, low pH is not an important metabolic signal for the liver enzyme, because lactate is not normally produced in the liver. Indeed, as we will see, lactate is converted into glucose in the liver.
Glycolysis in the liver furnishes carbon skeletons for biosyntheses, and so a signal indicating whether building blocks are abundant or scarce should also regulate phosphofructokinase. In the liver, phosphofructokinase is inhibited by citrate, an early intermediate in the citric acid cycle (Chapter 17). A high level of citrate in the cytoplasm means that biosynthetic precursors are abundant, and so there is no need to degrade additional glucose for this purpose. Citrate inhibits phosphofructokinase by enhancing the inhibitory effect of ATP.
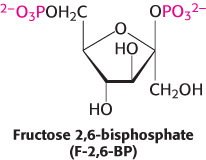
The key means by which glycolysis in the liver responds to changes in blood glucose is through the signal molecule fructose 2,6-bisphosphate (F-2,6-BP), a potent activator of phosphofructokinase (Figure 16.19). In the liver, the concentration of fructose 6-phosphate rises when blood-glucose concentration is high, and the abundance of fructose 6-phosphate accelerates the synthesis of F-2,6-BP (Figure 16.20). Hence, an abundance of fructose 6-phosphate leads to a higher concentration of F-2,6-BP. The binding of fructose 2,6-bisphosphate increases the affinity of phosphofructokinase for fructose 6-phosphate and diminishes the inhibitory effect of ATP. Glycolysis is thus accelerated when glucose is abundant. Such a process is called feedforward stimulation. We will turn to the synthesis and degradation of this important regulatory molecule after we have considered gluconeogenesis.
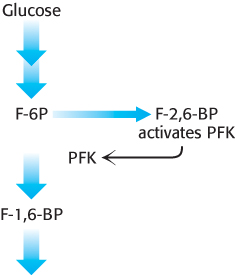
Figure 16.19: Regulation of phosphofructokinase by fructose 2,6-bisphosphate. In high concentrations, fructose 6-phosphate (F-6P) activates the enzyme phosphofructokinase (PFK) through an intermediary, fructose 2,6-bisphosphate (F-2,6-BP).
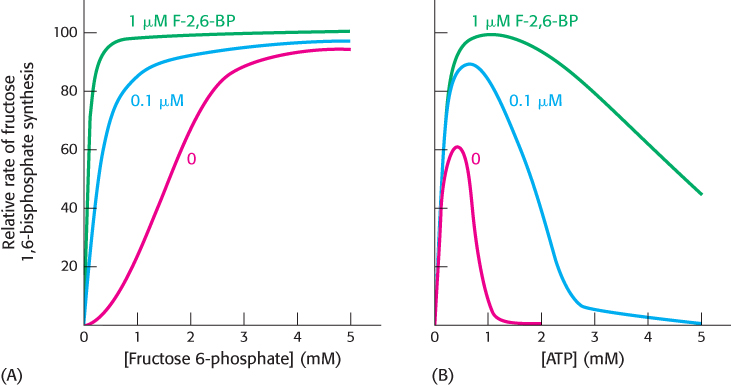
Figure 16.20: Activation of phosphofructokinase by fructose 2,6-bisphosphate. (A) The sigmoidal dependence of velocity on substrate concentration becomes hyperbolic in the presence of 1 μM fructose 2,6-bisphosphate. (B) ATP, acting as a substrate, initially stimulates the reaction. As the concentration of ATP increases, it acts as an allosteric inhibitor. The inhibitory effect of ATP is reversed by fructose 2,6-bisphosphate.
[Data from E. Van Schaftingen, M. F. Jett, L. Hue, and H. G. Hers, Proc. Natl. Acad. Sci. U. S. A. 78:3483–3486, 1981.]
Hexokinase and glucokinase.
The hexokinase reaction in the liver is controlled as in the muscle. However, the liver, in keeping with its role as monitor of blood-glucose levels, possesses another specialized isozyme of hexokinase, called glucokinase, which is not inhibited by glucose 6-phosphate. The role of glucokinase is to provide glucose 6-phosphate for the synthesis of glycogen and for the formation of fatty acids (Section 22.1). Remarkably, glucokinase displays the sigmoidal kinetics characteristic of an allosteric enzyme even though it functions as a monomer. Glucokinase phosphorylates glucose only when glucose is abundant because the affinity of glucokinase for glucose is about 50-fold lower than that of hexokinase. Moreover, when glucose concentration is low, glucokinase is inhibited by the liver-specific glucokinase regulatory protein. The low affinity of glucokinase for glucose gives the brain and muscles first call on glucose when its supply is limited, and it ensures that glucose will not be wasted when it is abundant. Drugs that activate liver glucokinase are being evaluated as a treatment for type 2 or insulin-insensitive diabetes. Glucokinase is also present in the β cells of the pancreas, where the increased formation of glucose 6-phosphate by glucokinase when blood-glucose levels are elevated leads to the secretion of the hormone insulin. Insulin signals the need to remove glucose from the blood for storage as glycogen or conversion into fat.
Pyruvate kinase.
Several isozymic forms of pyruvate kinase (a tetramer of 57-kDa subunits) encoded by different genes are present in mammals: the L type predominates in the liver, and the M type in muscle and the brain. The L and M forms of pyruvate kinase have many properties in common. Indeed, the liver enzyme behaves much like the muscle enzyme with regard to allosteric regulation except that the liver enzyme is also inhibited by alanine (synthesized in one step from pyruvate), a signal that building-blocks are available. Moreover, the isozymic forms differ in their susceptibility to covalent modification. The catalytic properties of the L form—but not of the M form—are also controlled by reversible phosphorylation (Figure 16.21). When the blood-glucose level is low, the glucagon-triggered cyclic AMP cascade leads to the phosphorylation of pyruvate kinase, which diminishes its activity. This hormone-triggered phosphorylation prevents the liver from consuming glucose when it is more urgently needed by the brain and muscle. We see here a clear-cut example of how isoenzymes contribute to the metabolic diversity of different organs. We will return to the control of glycolysis after considering gluconeogenesis.
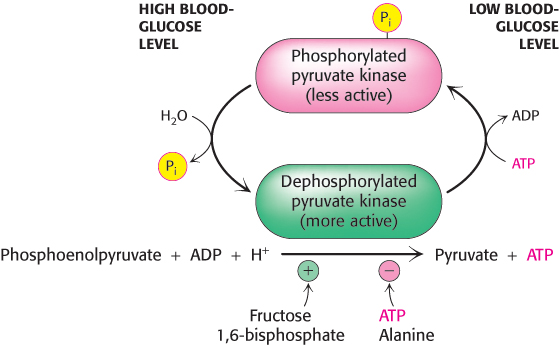
Figure 16.21: Control of the catalytic activity of pyruvate kinase. Pyruvate kinase is regulated by allosteric effectors and covalent modification. Fructose 1,6-bisphosphate allosterically stimulates the enzyme, while ATP and alanine are allosteric inhibitors. Glucagon, secreted in response to low blood glucose, promotes phosphorylation and inhibition of the enzyme. When blood glucose levels are adequate, the enzyme is dephosphorylated and activated.
A family of transporters enables glucose to enter and leave animal cells
Several glucose transporters mediate the thermodynamically downhill movement of glucose across the plasma membranes of animal cells. Each member of this protein family, named GLUT1 to GLUT5, consists of a single polypeptide chain about 500 residues long (Table 16.4). Each glucose transporter has a 12-transmembrane-helix structure similar to that of lactose permease (Section 13.3).
|
|
|
|
|
|
|
|
|
|
|
|
Liver and pancreatic β cells |
|
In the pancreas, plays a role in the regulation of insulin In the liver, removes excess glucose from the blood |
|
|
|
|
|
|
|
|
|
Amount in muscle plasma membrane increases with endurance training |
|
|
|
|
Primarily a fructose transporter |
Table 16.4: Family of glucose transporters
The members of this family have distinctive roles:
GLUT1 and GLUT3, present in nearly all mammalian cells, are responsible for basal glucose uptake. Their KM value for glucose is about 1 mM, significantly less than the normal serum-glucose level, which typically ranges from 4 mM to 8 mM. Hence, GLUT1 and GLUT3 continually transport glucose into cells at an essentially constant rate.
GLUT2, present in liver and pancreatic β cells, is distinctive in having a very high KM value for glucose (15–20 mM). Hence, glucose enters these tissues at a biologically significant rate only when there is much glucose in the blood. The pancreas can sense the glucose level and accordingly adjust the rate of insulin secretion. The high KM value of GLUT2 also ensures that glucose rapidly enters liver cells only in times of plenty.
GLUT4, which has a KM value of 5 mM, transports glucose into muscle and fat cells. The number of GLUT4 transporters in the plasma membrane increases rapidly in the presence of insulin, which signals the fed state. Hence, insulin promotes the uptake of glucose by muscle and fat. Endurance exercise training increases the amount of this transporter present in muscle membranes.
GLUT5, present in the small intestine, functions primarily as a fructose transporter.
Aerobic glycolysis is a property of rapidly growing cells.
 Tumors have been known for decades to display enhanced rates of glucose uptake and glycolysis. Indeed, rapidly growing tumor cells will metabolize glucose to lactate even in the presence of oxygen, a process called aerobic glycolysis or the Warburg effect, after Otto Warburg, the biochemist who first noted this characteristic of cancer cells in the 1920s. In fact, tumors with a high glucose uptake are particularly aggressive, and the cancer is likely to have a poor prognosis. A nonmetabolizable glucose analog, 2-18F-2-D-deoxyglucose, detectable by a combination of positron emission tomography (PET) and computer-aided tomography (CAT), easily visualizes tumors and allows monitoring of the effectiveness of treatment (Figure 16.22).
Tumors have been known for decades to display enhanced rates of glucose uptake and glycolysis. Indeed, rapidly growing tumor cells will metabolize glucose to lactate even in the presence of oxygen, a process called aerobic glycolysis or the Warburg effect, after Otto Warburg, the biochemist who first noted this characteristic of cancer cells in the 1920s. In fact, tumors with a high glucose uptake are particularly aggressive, and the cancer is likely to have a poor prognosis. A nonmetabolizable glucose analog, 2-18F-2-D-deoxyglucose, detectable by a combination of positron emission tomography (PET) and computer-aided tomography (CAT), easily visualizes tumors and allows monitoring of the effectiveness of treatment (Figure 16.22).
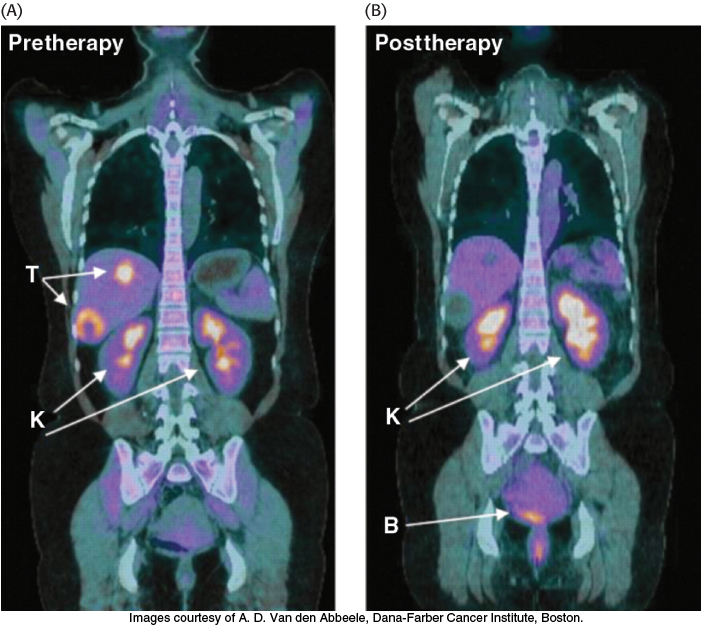
Figure 16.22: Tumors can be visualized with 2-18F-2-D-deoxyglucose (FDG) and positron emission tomography. (A) A nonmetabolizable glucose analog infused into a patient and detected by a combination of positron emission and computer-aided tomography reveals the presence of a malignant tumor (T). (B) After 4 weeks of treatment with a tyrosine kinase inhibitor (Section 14.5), the tumor shows no uptake of FDG, indicating decreased metabolism. Excess FDG, which is excreted in the urine, also visualizes the kidneys (K) and bladder (B).
[Images courtesy of A. D. Van den Abbeele, Dana-Farber Cancer Institute, Boston.]
What selective advantage does aerobic glycolysis offer the tumor over the energetically more efficient oxidative phosphorylation? Research is being actively pursued to answer the question, but we can speculate on the benefits. First, aerobic glycolysis generates lactic acid that is then secreted. Acidification of the tumor environment has been shown to facilitate tumor invasion and inhibit the immune system from attacking the tumor. However, even leukemias perform aerobic glycolysis, and leukemia is not an invasive cancer. Second, and perhaps more importantly, the increased uptake of glucose and formation of glucose 6-phosphate provides substrates for another metabolic pathway, the pentose phosphate pathway (Chapter 20), that generates biosynthetic reducing power. Moreover, the pentose phosphate pathway, in cooperation with glycolysis, produces precursors for biomolecules necessary for growth, such as nucleotides. Finally, cancer cells grow more rapidly than the blood vessels that nourish them; thus, as solid tumors grow, the oxygen concentration in their environment falls. In other words, they begin to experience hypoxia, a deficiency of oxygen. The use of aerobic glycolysis reduces the dependence of cell growth on oxygen. Not all of the precursor needs are met by enhanced glucose metabolism. Cancer cells also require glutamine, which is channeled into the mitochondria to replenish citric acid cycle components used for biosynthesis.
What biochemical alterations facilitate the switch to aerobic glycolysis? Again, the answers are not complete, but changes in gene expression of isozymic forms of two glycolytic enzymes may be crucial. Tumor cells express an isozyme of hexokinase that binds to mitochondria. There, the enzyme has ready access to any ATP generated by oxidative phosphorylation and is not susceptible to feedback inhibition by its product, glucose 6-phosphate. More importantly, an embryonic isozyme of pyruvate kinase, pyruvate kinase M, is also expressed. Remarkably, this isozyme has a lower catalytic rate than normal pyruvate kinase and creates a bottleneck, allowing the use of glycolytic intermediates for biosynthetic processes required for cell proliferation. The need for biosynthetic precursors is greater than the need for ATP, suggesting that even glycolysis at a reduced rate produces sufficient ATP to allow cell proliferation. Although originally observed in cancer cells, the Warburg effect is also seen in noncancerous, rapidly dividing cells.
Cancer and endurance training affect glycolysis in a similar fashion
|
|
|
|
|
|
|
|
|
|
Glyceraldehyde 3-phosphate dehydrogenase |
|
|
|
|
|
|
Table 16.5: Proteins in glucose metabolism encoded by genes regulated by hypoxia-inducible factor
The hypoxia that some tumors experience with rapid growth activates a transcription factor, hypoxia-inducible transcription factor (HIF-1). HIF-1 increases the expression of most glycolytic enzymes and the glucose transporters GLUT1 and GLUT3 (Table 16.5). These adaptations by the cancer cells enable a tumor to survive until blood vessels can grow. HIF-1 also increases the expression of signal molecules, such as vascular endothelial growth factor (VEGF), that facilitate the growth of blood vessels that will provide nutrients to the cells (Figure 16.23). Without new blood vessels, a tumor would cease to grow and either die or remain harmlessly small. Efforts are underway to develop drugs that inhibit the growth of blood vessels in tumors.
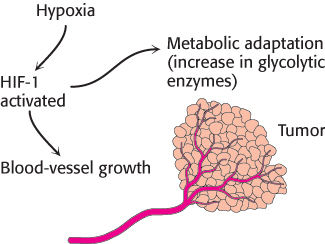
Figure 16.23: Alteration of gene expression in tumors owing to hypoxia. The hypoxic conditions inside a tumor mass lead to the activation of the hypoxia-inducible transcription factor (HIF-1), which induces metabolic adaptation (an increase in glycolytic enzymes) and activates angiogenic factors that stimulate the growth of new blood vessels.
[Information from C. V. Dang and G. L. Semenza, Trends Biochem. Sci. 24:68–72, 1999.]
Interestingly, anaerobic exercise training—forcing muscles to rely on lactic acid fermentation for ATP production—also activates HIF-1, producing the same effects as those seen in the tumor—enhanced ability to generate ATP anaerobically and a stimulation of blood-vessel growth. These biochemical effects account for the improved athletic performance that results from training and demonstrate how behavior can affect biochemistry. Other signals from sustained muscle contraction trigger muscle mitochondrial biogenesis, allowing for more efficient aerobic energy generation and forestalling the need to resort to lactic acid fermentation for ATP synthesis (Chapter 27).

 Structure of phosphofructokinase. The structure of phosphofructokinase from E. coli comprises a tetramer of four identical subunits. Notice the separation of the catalytic and allosteric sites. Each subunit of the human liver enzyme consists of two domains that are similar to the E. coli enzyme.
Structure of phosphofructokinase. The structure of phosphofructokinase from E. coli comprises a tetramer of four identical subunits. Notice the separation of the catalytic and allosteric sites. Each subunit of the human liver enzyme consists of two domains that are similar to the E. coli enzyme.







 Tumors have been known for decades to display enhanced rates of glucose uptake and glycolysis. Indeed, rapidly growing tumor cells will metabolize glucose to lactate even in the presence of oxygen, a process called aerobic glycolysis or the Warburg effect, after Otto Warburg, the biochemist who first noted this characteristic of cancer cells in the 1920s. In fact, tumors with a high glucose uptake are particularly aggressive, and the cancer is likely to have a poor prognosis. A nonmetabolizable glucose analog, 2-
Tumors have been known for decades to display enhanced rates of glucose uptake and glycolysis. Indeed, rapidly growing tumor cells will metabolize glucose to lactate even in the presence of oxygen, a process called aerobic glycolysis or the Warburg effect, after Otto Warburg, the biochemist who first noted this characteristic of cancer cells in the 1920s. In fact, tumors with a high glucose uptake are particularly aggressive, and the cancer is likely to have a poor prognosis. A nonmetabolizable glucose analog, 2-
