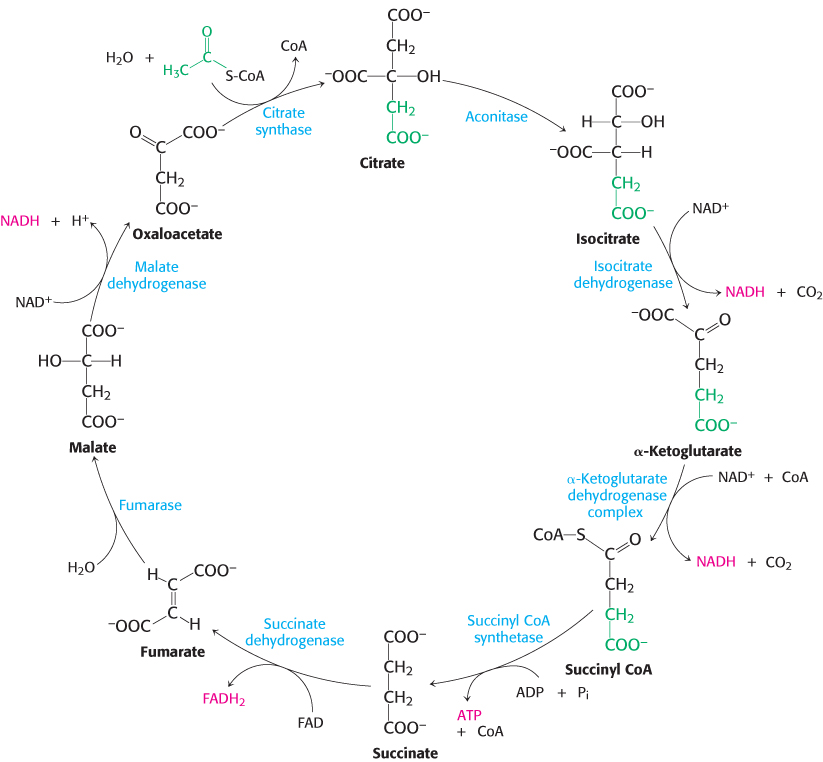
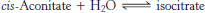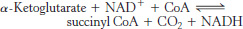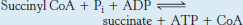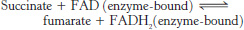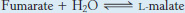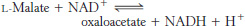17.2 The Citric Acid Cycle Oxidizes Two-Carbon Units
The conversion of pyruvate into acetyl CoA by the pyruvate dehydrogenase complex is the link between glycolysis and cellular respiration because acetyl CoA is the fuel for the citric acid cycle. Indeed, all fuels are ultimately metabolized to acetyl CoA or components of the citric acid cycle.
502
Citrate synthase forms citrate from oxaloacetate and acetyl coenzyme A
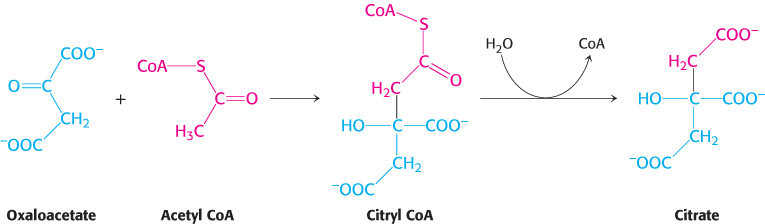
The citric acid cycle begins with the condensation of a four-
Synthase
An enzyme catalyzing a synthetic reaction in which two units are joined usually without the direct participation of ATP (or another nucleoside triphosphate).
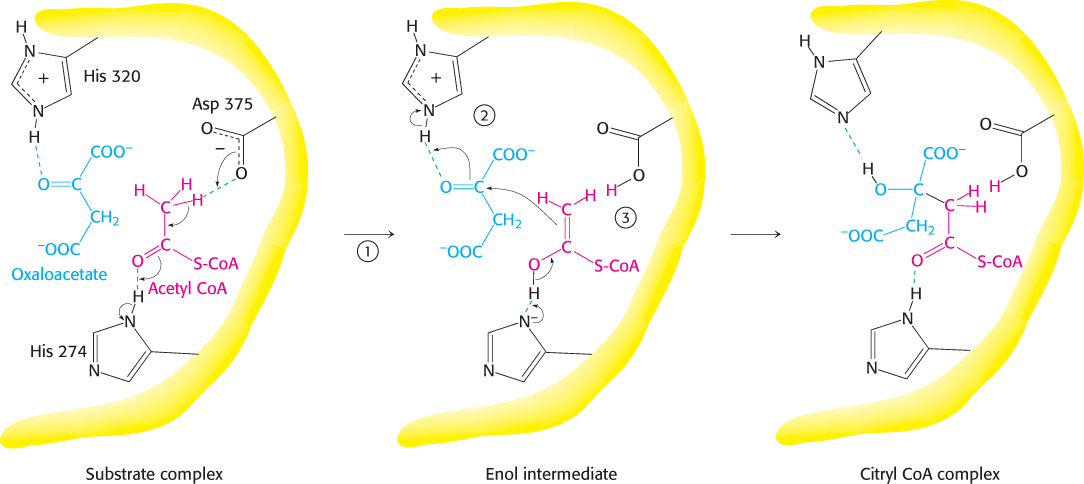
This reaction, which is an aldol condensation followed by a hydrolysis, is catalyzed by citrate synthase. Oxaloacetate first condenses with acetyl CoA to form citryl CoA, a molecule that is energy rich because it contains the thioester bond that originated in acetyl CoA. The hydrolysis of citryl CoA thioester to citrate and CoA drives the overall reaction far in the direction of the synthesis of citrate. In essence, the hydrolysis of the thioester powers the synthesis of a new molecule from two precursors.
Mechanism: The mechanism of citrate synthase prevents undesirable reactions
Because the condensation of acetyl CoA and oxaloacetate initiates the citric acid cycle, it is very important that side reactions, notably the hydrolysis of acetyl CoA to acetate and CoA, be minimized. Let us briefly consider how the citrate synthase prevents the wasteful hydrolysis of acetyl CoA.
Mammalian citrate synthase is a dimer of identical 49-
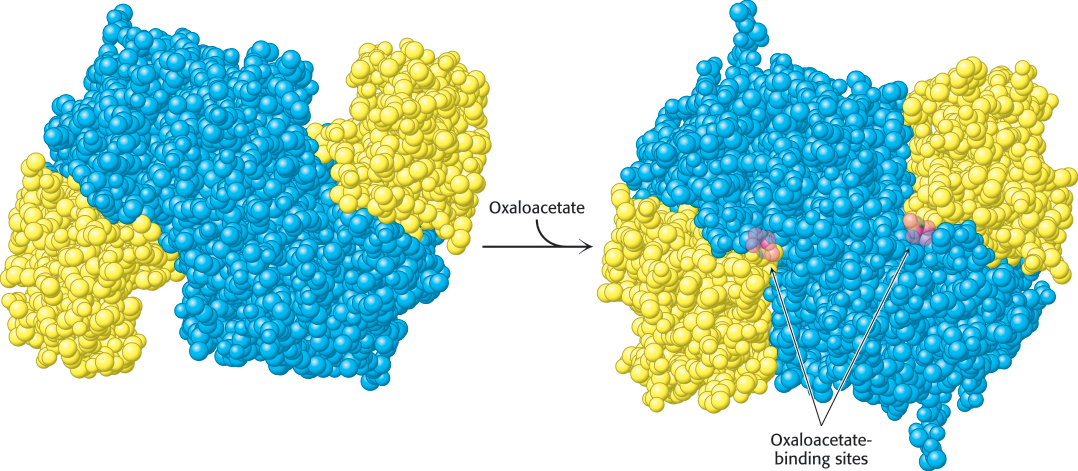
 Conformational changes in citrate synthase on binding oxaloacetate. The small domain of each subunit of the homodimer is shown in yellow; the large domains are shown in blue. (Left) Open form of enzyme alone. (Right) Closed form of the oxaloacetate-
Conformational changes in citrate synthase on binding oxaloacetate. The small domain of each subunit of the homodimer is shown in yellow; the large domains are shown in blue. (Left) Open form of enzyme alone. (Right) Closed form of the oxaloacetate-Citrate synthase catalyzes the condensation reaction by bringing the substrates into close proximity, orienting them, and polarizing certain bonds (Figure 17.11). The donation and removal of protons transforms acetyl CoA into an enol intermediate. The enol attacks oxaloacetate to form a carbon−carbon double bond linking acetyl CoA and oxaloacetate. The newly formed citryl CoA induces additional structural changes in the enzyme, causing the active site to become completely enclosed. The enzyme cleaves the citryl CoA thioester by hydrolysis. CoA leaves the enzyme, followed by citrate, and the enzyme returns to the initial open conformation.
503
We can now understand how the wasteful hydrolysis of acetyl CoA is prevented. Citrate synthase is well suited to hydrolyze citryl CoA but not acetyl CoA. How is this discrimination accomplished? First, acetyl CoA does not bind to the enzyme until oxaloacetate is bound and ready for condensation. Second, the catalytic residues crucial for the hydrolysis of the thioester linkage are not appropriately positioned until citryl CoA is formed. As with hexokinase and triose phosphate isomerase (Section 16.1), induced fit prevents an undesirable side reaction.
504
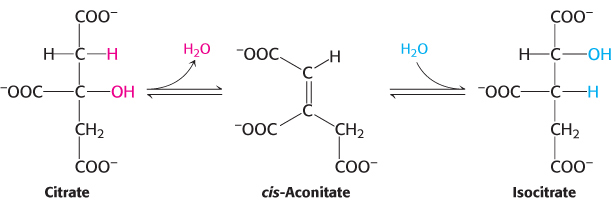
Citrate is isomerized into isocitrate
The hydroxyl group is not properly located in the citrate molecule for the oxidative decarboxylations that follow (Problem 27). Thus, citrate is isomerized into isocitrate to enable the six-
Aconitase is an iron–
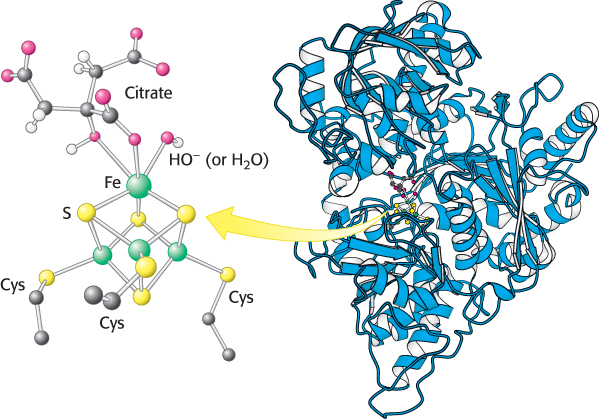
 Binding of citrate to the iron–
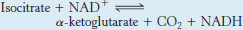
Binding of citrate to the iron–Isocitrate is oxidized and decarboxylated to alpha-ketoglutarate
We come now to the first of four oxidation–
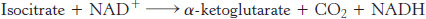
The intermediate in this reaction is oxalosuccinate, an unstable β-ketoacid. While bound to the enzyme, it loses CO2 to form α-ketoglutarate.
505
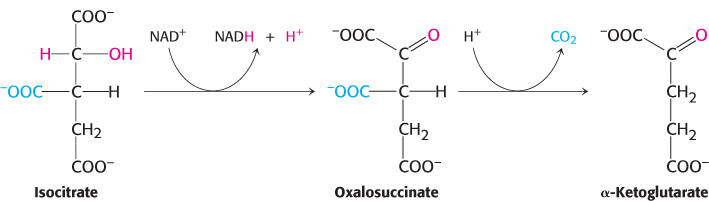
This oxidation generates the first high-
Succinyl coenzyme A is formed by the oxidative decarboxylation of alpha-ketoglutarate
The conversion of isocitrate into α-ketoglutarate is followed by a second oxidative decarboxylation reaction, the formation of succinyl CoA from α-ketoglutarate.
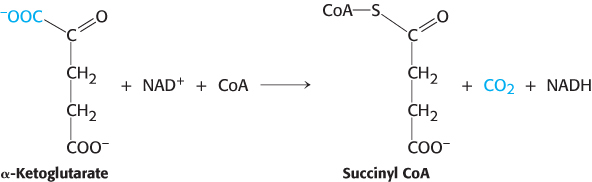
 This reaction is catalyzed by the α-ketoglutarate dehydrogenase complex, an organized assembly of three kinds of enzymes that is homologous to the pyruvate dehydrogenase complex. In fact, the E3 component is identical in both enzymes. The oxidative decarboxylation of α-ketoglutarate closely resembles that of pyruvate, also an α-ketoacid.
This reaction is catalyzed by the α-ketoglutarate dehydrogenase complex, an organized assembly of three kinds of enzymes that is homologous to the pyruvate dehydrogenase complex. In fact, the E3 component is identical in both enzymes. The oxidative decarboxylation of α-ketoglutarate closely resembles that of pyruvate, also an α-ketoacid.
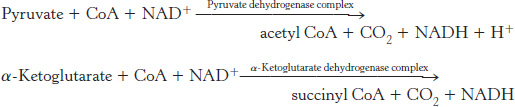
Both reactions include the decarboxylation of an α-ketoacid and the subsequent formation of a thioester linkage with CoA that has a high transfer potential. The reaction mechanisms are entirely analogous.
A compound with high phosphoryl-transfer potential is generated from succinyl coenzyme A
Succinyl CoA is an energy-
506
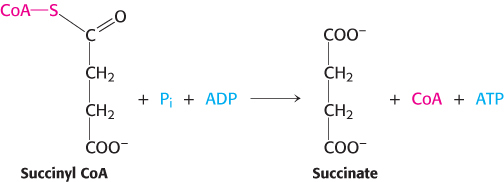
This reaction is the only step in the citric acid cycle that directly yields a compound with high phosphoryl-
Note that the enzyme nucleoside diphosphokinase, which catalyzes the following reaction,

allows the γ phosphoryl group to be readily transferred from GTP to form ATP, thereby allowing the adjustment of the concentration of GTP or ATP to meet the cell’s need.
Mechanism: Succinyl coenzyme A synthetase transforms types of biochemical energy
The mechanism of this reaction is a clear example of an energy transformation: energy inherent in the thioester molecule is transformed into phosphoryl-
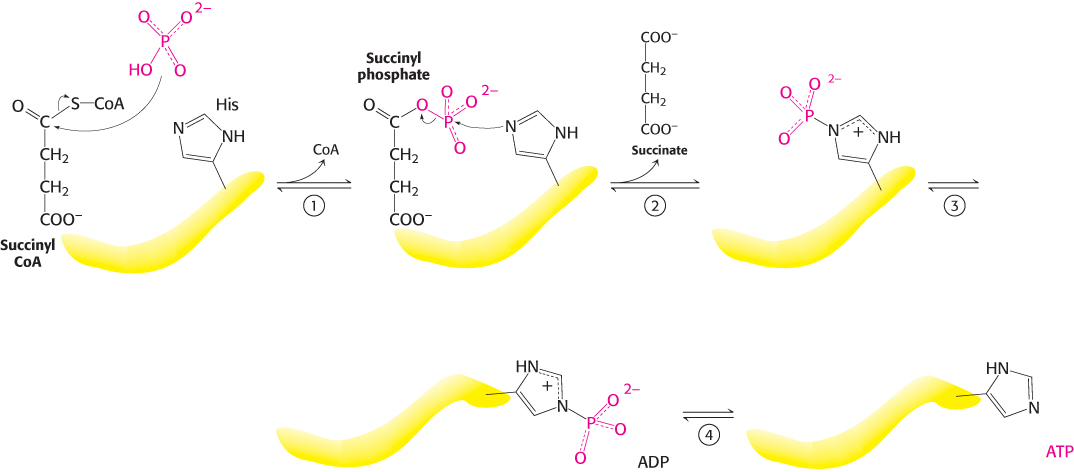
507
 Succinyl CoA synthetase is an α2β2 heterodimer; the functional unit is one αβ pair. The enzyme mechanism shows that a phosphoryl group is transferred first to succinyl CoA bound in the α subunit and then to a nucleoside diphosphate bound in the β subunit. Examination of the three-
Succinyl CoA synthetase is an α2β2 heterodimer; the functional unit is one αβ pair. The enzyme mechanism shows that a phosphoryl group is transferred first to succinyl CoA bound in the α subunit and then to a nucleoside diphosphate bound in the β subunit. Examination of the three-
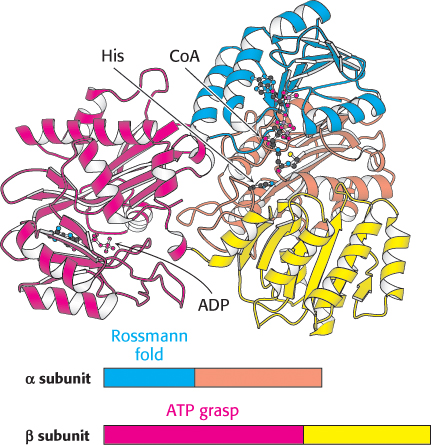
 Structure of succinyl CoA synthetase. The enzyme is composed of two subunits. The α subunit contains a Rossmann fold that binds the ADP component of CoA, and the β subunit contains a nucleotide-
Structure of succinyl CoA synthetase. The enzyme is composed of two subunits. The α subunit contains a Rossmann fold that binds the ADP component of CoA, and the β subunit contains a nucleotide-Oxaloacetate is regenerated by the oxidation of succinate
Reactions of four-
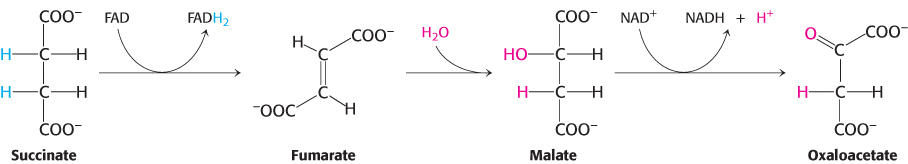
The reactions constitute a metabolic motif that we will see again in fatty acid synthesis and degradation as well as in the degradation of some amino acids. A methylene group (CH2) is converted into a carbonyl group CPO in three steps: an oxidation, a hydration, and a second oxidation reaction. Oxaloacetate is thereby regenerated for another round of the cycle, and more energy is extracted in the form of FADH2 and NADH.
Succinate is oxidized to fumarate by succinate dehydrogenase. The hydrogen acceptor is FAD rather than NAD+, which is used in the other three oxidation reactions in the cycle. FAD is the hydrogen acceptor in this reaction because the free-

508
Succinate dehydrogenase, like aconitase, is an iron–
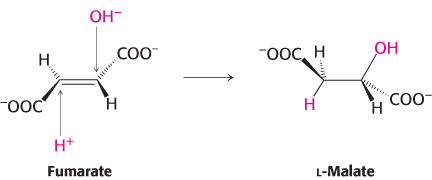
The next step is the hydration of fumarate to form l-malate. Fumarase catalyzes a stereospecific trans addition of H+ and OH−. The OH− group adds to only one side of the double bond of fumarate; hence, only the L isomer of malate is formed.
Finally, malate is oxidized to form oxaloacetate. This reaction is catalyzed by malate dehydrogenase, and NAD+ is again the hydrogen acceptor.
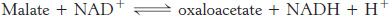
The standard free energy for this reaction, unlike that for the other steps in the citric acid cycle, is significantly positive (ΔG°′ = +29.7 kJ mol−1, or +7.1 kcal mol−1). The oxidation of malate is driven by the use of the products—
The citric acid cycle produces high-
The net reaction of the citric acid cycle is
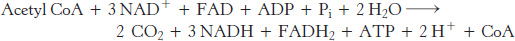
Let us recapitulate the reactions that give this stoichiometry (Figure 17.15 and Table 17.2):
Two carbon atoms enter the cycle in the condensation of an acetyl unit (from acetyl CoA) with oxaloacetate. Two carbon atoms leave the cycle in the form of CO2 in the successive decarboxylations catalyzed by isocitrate dehydrogenase and α-ketoglutarate dehydrogenase.
Four pairs of hydrogen atoms leave the cycle in four oxidation reactions. Two NAD+ molecules are reduced in the oxidative decarboxylations of isocitrate and α-ketoglutarate, one FAD molecule is reduced in the oxidation of succinate, and one NAD+ molecule is reduced in the oxidation of malate. Recall also that one NAD+ molecule is reduced in the oxidative decarboxylation of pyruvate to form acetyl CoA.
509
510
One compound with high phosphoryl-
transfer potential, usually ATP, is generated from the cleavage of the thioester linkage in succinyl CoA. Two water molecules are consumed: one in the synthesis of citrate by the hydrolysis of citryl CoA and the other in the hydration of fumarate.
|
ΔG°′ |
||||||
|---|---|---|---|---|---|---|
|
Step |
Reaction |
Enzyme |
Prosthetic group |
Type* |
kJ mol−1 |
kcal mol−1 |
|
1 |
Acetyl CoA + oxaloacetate + H2O → citrate + CoA + H+ |
Citrate synthase |
a |
–31.4 |
–7.5 |
|
|
2a |
|
Aconitase |
Fe- |
b |
+8.4 |
+2.0 |
|
2b |
|
Aconitase |
Fe- |
c |
−2.1 |
−0.5 |
|
3 |
|
Isocitrate dehydrogenase |
d + e |
−8.4 |
−2.0 |
|
|
4 |
|
α-Ketoglutarate dehydrogenase complex |
Lipoic acid, FAD, TPP |
d + e |
−30.1 |
−7.2 |
|
5 |
|
Succinyl CoA synthetase |
f |
−3.3 |
−0.8 |
|
|
6 |
|
Succinate dehydrogenase |
FAD, Fe- |
e |
0 |
0 |
|
7 |
|
Fumarase |
c |
−3.8 |
−0.9 |
|
|
8 |
|
Malate dehydrogenase |
|
e |
+29.7 |
+7.1 |
|
*Reaction type: (a) condensation; (b) dehydration; (c) hydration; (d) decarboxylation; (e) oxidation; (f) substrate- |
||||||
Isotope-
Evidence is accumulating that the enzymes of the citric acid cycle are physically associated with one another. The close arrangement of enzymes enhances the efficiency of the citric acid cycle because a reaction product can pass directly from one active site to the next through connecting channels, a process called substrate channeling.
As will be considered in Chapter 18, the electron-
Recall that molecular oxygen does not participate directly in the citric acid cycle. However, the cycle operates only under aerobic conditions because NAD+ and FAD can be regenerated in the mitochondrion only by the transfer of electrons to molecular oxygen. Glycolysis has both an aerobic and an anaerobic mode, whereas the citric acid cycle is strictly aerobic. Glycolysis can proceed under anaerobic conditions because NAD+ is regenerated in the conversion of pyruvate into lactate or ethanol.