17.4 The Citric Acid Cycle Is a Source of Biosynthetic Precursors
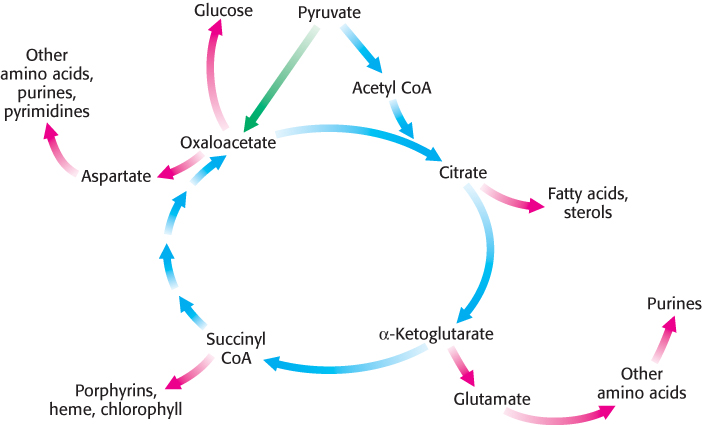
Thus far, discussion has focused on the citric acid cycle as the major degradative pathway for the generation of ATP. As a major metabolic hub of the cell, the citric acid cycle also provides intermediates for biosyntheses (Figure 17.20). For example, most of the carbon atoms in porphyrins come from succinyl CoA. Many of the amino acids are derived from α-ketoglutarate and oxaloacetate. These biosynthetic processes will be considered in subsequent chapters.
The citric acid cycle must be capable of being rapidly replenished
An important consideration is that citric acid cycle intermediates must be replenished if any are drawn off for biosyntheses. Suppose that much oxaloacetate is converted into amino acids for protein synthesis and, subsequently, the energy needs of the cell rise. The citric acid cycle will operate to a reduced extent unless new oxaloacetate is formed because acetyl CoA cannot enter the cycle unless it condenses with oxaloacetate. Even though oxaloacetate is recycled, a minimal level must be maintained to allow the cycle to function.
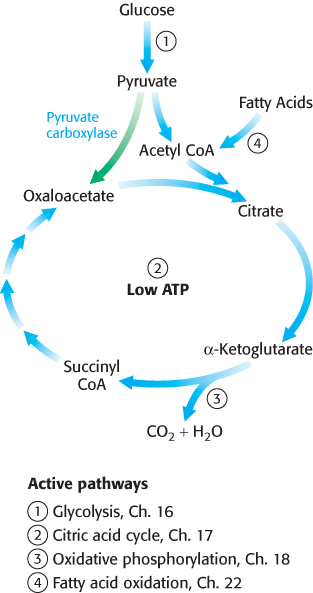
How is oxaloacetate replenished? Mammals lack the enzymes for the net conversion of acetyl CoA into oxaloacetate or any other citric acid cycle intermediate. Rather, oxaloacetate is formed by the carboxylation of pyruvate, in a reaction catalyzed by the biotindependent enzyme pyruvate carboxylase (Figure 17.21).
Pyruvate + CO2 + ATP + H2O → oxaloacetate + ADP + Pi + 2H+
515
Recall that this enzyme plays a crucial role in gluconeogenesis (Section 16.3). It is active only in the presence of acetyl CoA, which signifies the need for more oxaloacetate. If the energy charge is high, oxaloacetate is converted into glucose. If the energy charge is low, oxaloacetate replenishes the citric acid cycle. The synthesis of oxaloacetate by the carboxylation of pyruvate is an example of an anaplerotic reaction (anaplerotic is of Greek origin, meaning to “fill up”), a reaction that leads to the net synthesis, or replenishment, of pathway components. Note that because the citric acid cycle is a cycle, it can be replenished by the generation of any of the intermediates. Glutamine is an especially important source of citric acid cycle intermediates in rapidly growing cells, including cancer cells. Glutamine is converted into glutamate and then into α-ketoglutarate.
The disruption of pyruvate metabolism is the cause of beriberi and poisoning by mercury and arsenic
Beriberi
A vitamin-
“A certain very troublesome affliction, which attacks men, is called by the inhabitants Beriberi (which means sheep). I believe those, whom this same disease attacks, with their knees shaking and the legs raised up, walk like sheep. It is a kind of paralysis, or rather Tremor: for it penetrates the motion and sensation of the hands and feet indeed sometimes of the whole body.”
 Beriberi, a neurologic and cardiovascular disorder, is caused by a dietary deficiency of thiamine (also called vitamin B1). The disease has been and continues to be a serious health problem in the Far East because rice, the major food, has a rather low content of thiamine. This deficiency is partly ameliorated if the whole rice grain is soaked in water before milling; some of the thiamine in the husk then leaches into the rice kernel. The problem is exacerbated if the rice is polished to remove the outer layer (that is, converted from brown to white rice), because only the outer layer contains significant amounts of thiamine. A form of beriberi, called Wernicke’s encephalopathy, is also occasionally seen in alcoholics who are severely malnourished and thus thiamine deficient. The disease is characterized by neurologic and cardiac symptoms. Damage to the peripheral nervous system is expressed as pain in the limbs, weakness of the musculature, and distorted skin sensation. The heart may be enlarged and the cardiac output inadequate.
Beriberi, a neurologic and cardiovascular disorder, is caused by a dietary deficiency of thiamine (also called vitamin B1). The disease has been and continues to be a serious health problem in the Far East because rice, the major food, has a rather low content of thiamine. This deficiency is partly ameliorated if the whole rice grain is soaked in water before milling; some of the thiamine in the husk then leaches into the rice kernel. The problem is exacerbated if the rice is polished to remove the outer layer (that is, converted from brown to white rice), because only the outer layer contains significant amounts of thiamine. A form of beriberi, called Wernicke’s encephalopathy, is also occasionally seen in alcoholics who are severely malnourished and thus thiamine deficient. The disease is characterized by neurologic and cardiac symptoms. Damage to the peripheral nervous system is expressed as pain in the limbs, weakness of the musculature, and distorted skin sensation. The heart may be enlarged and the cardiac output inadequate.
Which biochemical processes might be affected by a deficiency of thiamine? Thiamine is the precursor of the cofactor thiamine pyrophosphate. This cofactor is the prosthetic group of three important enzymes: pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and transketolase. Transketolase functions in the pentose phosphate pathway, which will be considered in Chapter 20. The common feature of enzymatic reactions utilizing TPP is the transfer of an activated aldehyde unit. In beriberi, the levels of pyruvate and α-ketoglutarate in the blood are higher than normal. The increase in the level of pyruvate in the blood is especially pronounced after the ingestion of glucose. A related finding is that the activities of the pyruvate and α-ketoglutarate dehydrogenase complexes in vivo are abnormally low. The low transketolase activity of red blood cells in beriberi is an easily measured and reliable diagnostic indicator of the disease.

Why does TPP deficiency lead primarily to neurological disorders? The nervous system relies essentially on glucose as its only fuel. The product of glycolysis, pyruvate, can enter the citric acid cycle only through the pyruvate dehydrogenase complex. With that enzyme deactivated, the nervous system has no source of fuel. In contrast, most other tissues can use fats as a source of fuel for the citric acid cycle.
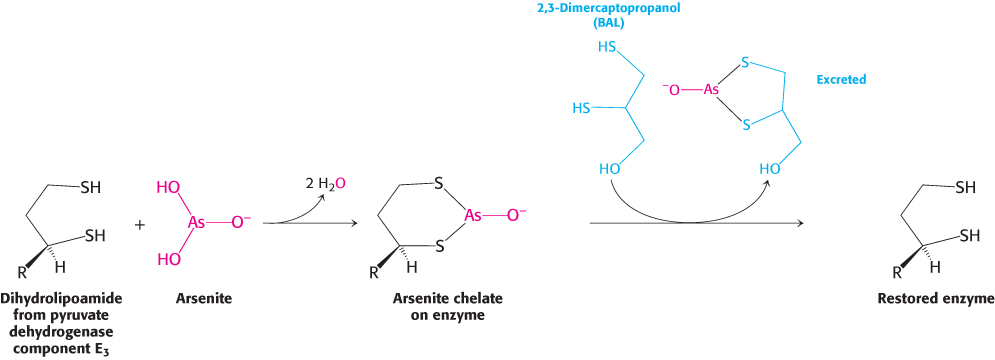
Symptoms similar to those of beriberi appear in organisms exposed to mercury or arsenite (AsO33−). Both materials have a high affinity for neighboring sulfhydryls, such as those in the reduced dihydrolipoyl groups of the E3 component of the pyruvate dehydrogenase complex (Figure 17.22). The binding of mercury or arsenite to the dihydrolipoyl groups inhibits the complex and leads to central nervous system pathologies. The proverbial phrase “mad as a hatter” refers to the strange behavior of poisoned hat makers who used mercury nitrate to soften and shape animal furs. This form of mercury is absorbed through the skin. Similar symptoms afflicted the early photographers, who used vaporized mercury to create daguerreotypes.
516
Treatment for these poisons is the administration of sulfhydryl reagents with adjacent sulfhydryl groups to compete with the dihydrolipoyl residues for binding with the metal ion. The reagent–
The citric acid cycle may have evolved from preexisting pathways
The manuscript proposing the citric acid cycle was submitted for publication to Nature but was rejected in June 1937. That same year it was published in Enzymologia. Dr. Krebs proudly displayed the rejection letter throughout his career as encouragement for young scientists.
“The editor of NATURE presents his compliments to Dr. H. A. Krebs and regrets that as he has already sufficient letters to fill the correspondence columns of NATURE for seven or eight weeks, it is undesirable to accept further letters at the present time on account of the time delay which must occur in their publication.
If Dr. Krebs does not mind much delay the editor is prepared to keep the letter until the congestion is relieved in the hope of making use of it.
He returns it now, in case Dr. Krebs prefers to submit it for early publication to another periodical.”
 How did the citric acid cycle come into being? Although definitive answers are elusive, informed speculation is possible. We can perhaps begin to comprehend how evolution might work at the level of biochemical pathways.
How did the citric acid cycle come into being? Although definitive answers are elusive, informed speculation is possible. We can perhaps begin to comprehend how evolution might work at the level of biochemical pathways.
The citric acid cycle was most likely assembled from preexisting reaction pathways. As noted earlier, many of the intermediates formed in the citric acid cycle are used in metabolic pathways for amino acids and porphyrins. Thus, compounds such as pyruvate, α-ketoglutarate, and oxaloacetate were likely present early in evolution for biosynthetic purposes. The oxidative decarboxylation of these α-ketoacids is quite favorable thermodynamically and can be used to drive the synthesis of both acyl CoA derivatives and NADH. These reactions almost certainly formed the core of processes that preceded the citric acid cycle evolutionarily. Interestingly, α-ketoglutarate and oxaloacetate can be interconverted by transamination of the respective amino acids by aspartate aminotransferase, another key biosynthetic enzyme. Thus, cycles comprising smaller numbers of intermediates used for a variety of biochemical purposes could have existed before the present form evolved.