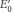18.2 Oxidative Phosphorylation Depends on Electron Transfer
In Chapter 17, the generation of NADH and FADH2 by the oxidation of acetyl CoA was identified as an important function of the citric acid cycle. In oxidative phosphorylation, electrons from NADH and FADH2 are used to reduce molecular oxygen to water. The highly exergonic reduction is accomplished by a number of electron-
The electron-transfer potential of an electron is measured as redox potential
In oxidative phosphorylation, the electron-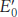 , the reduction potential (also called the redox potential or oxidation–
, the reduction potential (also called the redox potential or oxidation–
Consider a substance that can exist in an oxidized form X and a reduced form X−. Such a pair is called a redox couple and is designated X : X−. The reduction potential of this couple can be determined by measuring the electromotive force generated by an apparatus called a sample half-
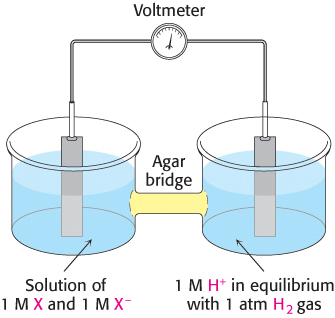
527
X− + H+ → X + ½ H2
the reactions in the half-
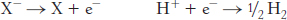
Thus, electrons flow from the sample half-
The meaning of the reduction potential is now evident. A negative reduction potential means that the oxidized form of a substance has lower affinity for electrons than does H2, as in the preceding example. A positive reduction potential means that the oxidized form of a substance has higher affinity for electrons than does H2. These comparisons refer to standard conditions—
The reduction potentials of many biologically important redox couples are known (Table 18.1). Table 18.1 is like those presented in chemistry textbooks, except that a hydrogen ion concentration of 10−7 M (pH 7) instead of 1 M (pH 0) is the standard state adopted by biochemists. This difference is denoted by the prime in 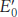 . Recall that the prime in ΔG°′ denotes a standard free-
. Recall that the prime in ΔG°′ denotes a standard free-
|
Oxidant |
Reductant |
n |
|
|---|---|---|---|
|
Succinate + CO2 |
α-Ketoglutarate |
2 |
−0.67 |
|
Acetate |
Acetaldehyde |
2 |
−0.60 |
|
Ferredoxin (oxidized) |
Ferredoxin (reduced) |
1 |
−0.43 |
|
2 H+ |
H2 |
2 |
−0.42 |
|
NAD+ |
NADH + H+ |
2 |
−0.32 |
|
NADP+ |
NADPH + H+ |
2 |
−0.32 |
|
Lipoate (oxidized) |
Lipoate (reduced) |
2 |
−0.29 |
|
Glutathione (oxidized) |
Glutathione (reduced) |
2 |
−0.23 |
|
FAD |
FADH2 |
2 |
−0.22 |
|
Acetaldehyde |
Ethanol |
2 |
−0.20 |
|
Pyruvate |
Lactate |
2 |
−0.19 |
|
2 H+ |
H2 |
2 |
−0.001 |
|
Fumarate |
Succinate |
2 |
+0.03 |
|
Cytochrome b (+3) |
Cytochrome b (+2) |
1 |
+0.07 |
|
Dehydroascorbate |
Ascorbate |
2 |
+0.08 |
|
Ubiquinone (oxidized) |
Ubiquinone (reduced) |
2 |
+0.10 |
|
Cytochrome c (+3) |
Cytochrome c (+2) |
1 |
+0.22 |
|
Fe (+3) |
Fe (+2) |
1 |
+0.77 |
|
½ O2 + 2 H+ |
H2O |
2 |
+0.82 |
|
Note: 1Standard oxidation − reduction potential at pH = 0. |
|||
528
The standard free-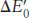 by
by

in which n is the number of electrons transferred, F is a proportionality constant called the Faraday constant [96.48 kJ mol−1V−1 (23.06 kcal mol−1 V−1)], 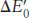 is in volts, and ΔG°′ is in kilojoules or kilocalories per mole.
is in volts, and ΔG°′ is in kilojoules or kilocalories per mole.
The free-

The reduction potential of the NAD+ : NADH couple, or half-
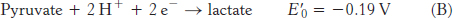
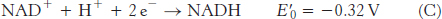
To obtain reaction A from reactions B and C, we need to reverse the direction of reaction C so that NADH appears on the left side of the arrow. In doing so, the sign of 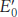 must be changed.
must be changed.
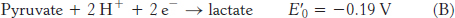
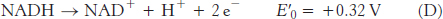
For reaction B, the free energy can be calculated with n = 2.
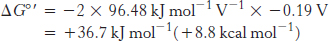
Likewise, for reaction D,
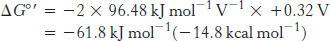
Thus, the free energy for reaction A is given by
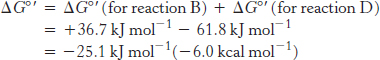
A 1.14-volt potential difference between NADH and molecular oxygen drives electron transport through the chain and favors the formation of a proton gradient
The driving force of oxidative phosphorylation is the electron-
529
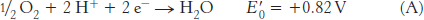
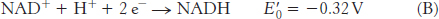
The combination of the two half-
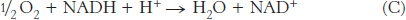
The standard free energy for this reaction is then given by
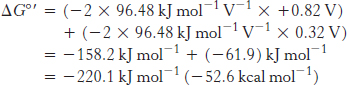
This release of free energy is substantial. Recall that ΔG°′ for the synthesis of ATP is 30.5 kJ mol−1 (7.3 kcal mol−1). The released energy is initially used to generate a proton gradient that is then used for the synthesis of ATP and the transport of metabolites across the mitochondrial membrane.
How can the energy associated with a proton gradient be quantified? Recall that the free-
ΔG = RT ln (c2/c1) + ZF Δ V
in which Z is the electrical charge of the transported species and ΔV is the potential in volts across the membrane (Section 13.1). Under typical conditions for the inner mitochondrial membrane, the pH outside is 1.4 units lower than inside [corresponding to ln (c2/c1) of 3.2] and the membrane potential is 0.14 V, the outside being positive. Because Z = +1 for protons, the free-