Chapter Introduction
The Light Reactions of Photosynthesis
565
OUTLINE
Photosynthesis Takes Place in Chloroplasts
Light Absorption by Chlorophyll Induces Electron Transfer
Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
A Proton Gradient across the Thylakoid Membrane Drives ATP Synthesis
Accessory Pigments Funnel Energy into Reaction Centers
The Ability to Convert Light into Chemical Energy Is Ancient
Every year, Earth is bathed in photons with a total energy content of approximately 1024 kJ. By comparison, a major hurricane has 10−9 that amount of energy. The source of the energy is, of course, the electromagnetic radiation from the sun. On our planet, there are organisms capable of collecting a fraction, approximately 1%, of this solar energy and converting it into chemical energy. Green plants are the most obvious of these organisms, although 60% of this conversion is carried out by algae and bacteria. Although only a small amount of the incident energy is captured, it is enough to power all life on Earth. This transformation is perhaps the most important of all of the energy transformations that we will see in our study of biochemistry; without it, life as we know it on our planet simply could not exist.
The process of converting electromagnetic radiation into chemical energy is called photosynthesis, which uses light energy to convert carbon dioxide and water into carbohydrates and oxygen.
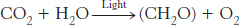
In this equation, CH2O represents carbohydrate, primarily sucrose and starch. Photosynthetic carbohydrate synthesis is the most common metabolic pathway on Earth. These carbohydrates provide not only the energy to run the biological world, but also the carbon molecules to make a wide array of biomolecules. Photosynthetic organisms are called autotrophs (literally, “self-
566
Photosynthesis is composed of two parts: the light reactions and the dark reactions. In the light reactions, light energy is transformed into two forms of biochemical energy with which we are already familiar: reducing power and ATP. The products of the light reactions are then used in the dark reactions to drive the reduction of CO2 and its conversion into glucose and other sugars. The dark reactions are also called the Calvin cycle or light-
Photosynthesis converts light energy into chemical energy
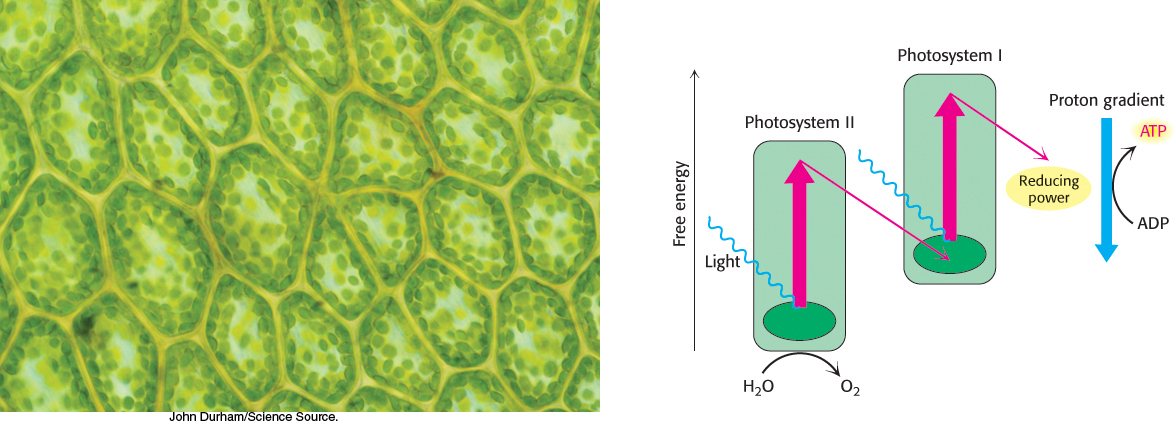
The light reactions of photosynthesis closely resemble the events of oxidative phosphorylation. In Chapters 17 and 18, we learned that cellular respiration is the oxidation of glucose to CO2 with the reduction of O2 to water, a process that generates ATP. In photosynthesis, this process must be reversed—
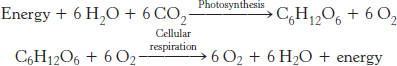
Photosynthetic yield
“If a year’s yield of photosynthesis were amassed in the form of sugar cane, it would form a heap over two miles high and with a base 43 square miles.”
—G. E. Fogge
If all of this sugar cane were converted into sugar cubes (0.5 inch, or 1.27 cm, on a side) and stacked end to end, the sugar cubes would extend 1.6 × 1010 miles (2.6 × 1010 kilometers) or to the dwarf planet Pluto.
Although the processes of respiration and photosynthesis are chemically opposite each other, the biochemical principles governing the two processes are nearly identical. The key to both processes is the generation of high-
Photosynthetic catastrophe
If photosynthesis were to cease, all higher forms of life would be extinct in about 25 years. A milder version of such a catastrophe ended the Cretaceous period 65.1 million years ago when a large asteroid struck the Yucatan Peninsula of Mexico. Enough dust was sent into the atmosphere that photosynthetic capacity was greatly diminished, which apparently led to the disappearance of the dinosaurs and allowed the mammals to rise to prominence.
567
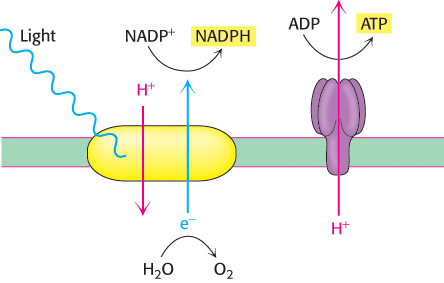
Photosynthesis in green plants is mediated by two kinds of light reactions. Photosystem I generates reducing power in the form of NADPH, but, in the process, becomes electron deficient. Photosystem II oxidizes water and transfers the electrons to replenish the electrons lost by photosystem I. A side product of these reactions is O2. Electron flow from photosystem II to photosystem I generates the transmembrane proton gradient, augmented by the protons released by the oxidation of water, that drives the synthesis of ATP. In keeping with the similarity of their principles of operation, both processes take place in double-