20.2 The Activity of the Calvin Cycle Depends on Environmental Conditions
How do the light reactions communicate with the dark reactions to regulate this crucial process of fixing CO2 into biomolecules? The principal means of regulation is alteration of the stromal environment by the light reactions. The light reactions lead to an increase in stromal pH (a decrease in the H+ concentration), as well as an increase in the stromal concentrations of Mg2+, NADPH, and reduced ferredoxin—changes that contribute to the activation of certain Calvin-cycle enzymes, located in the stroma (Figure 20.13).
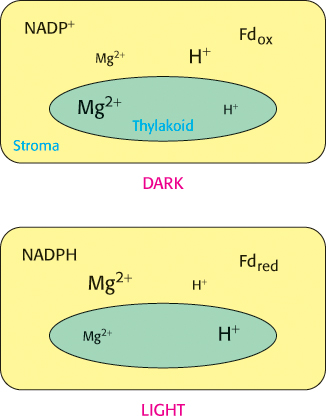
Figure 20.13: Light regulation of the Calvin cycle. The light reactions of photosynthesis transfer electrons out of the thylakoid lumen into the stroma and transfer protons from the stroma into the thylakoid lumen. As a consequence of these processes, the concentrations of NADPH, reduced ferredoxin (Fd), and Mg2+ in the stroma are higher in the light than in the dark. The stromal pH is also increased (the concentration of H+ is lowered) in the light as a result of proton pumping from the stroma to the thylakoid lumen. Each of these concentration changes helps couple the Calvin cycle reactions to the light reactions.
Rubisco is activated by light-driven changes in proton and magnesium ion concentrations
As stated earlier, the rate-limiting step in the Calvin cycle is the carboxylation of ribulose 1,5-bisphosphate to form two molecules of 3-phosphoglycerate. The activity of rubisco increases markedly on illumination because light facilitates the carbamate formation necessary for enzyme activity. In the stroma, the pH increases from 7 to 8, and the level of Mg2+rises. Both effects are consequences of the light-driven pumping of protons into the thylakoid space. Mg2+ ions from the thylakoid space are released into the stroma to compensate for the influx of protons. Carbamate formation is favored at alkaline pH. CO2 adds to a deprotonated form of lysine 201 of rubisco, and Mg2+ ion binds to the carbamate to generate the active form of the enzyme. Thus, light leads to the generation of regulatory signals as well as ATP and NADPH.
Thioredoxin plays a key role in regulating the Calvin cycle
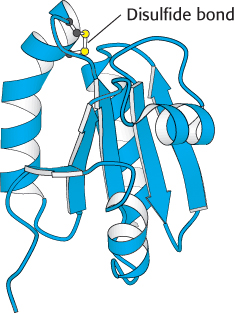
Figure 20.14:  Thioredoxin. The oxidized form of thioredoxin contains a disulfide bond. When thioredoxin is reduced by reduced ferredoxin, the disulfide bond is converted into two free sulfhydryl groups. Reduced thioredoxin can cleave disulfide bonds in enzymes, activating certain Calvin cycle enzymes and inactivating some degradative enzymes.
Thioredoxin. The oxidized form of thioredoxin contains a disulfide bond. When thioredoxin is reduced by reduced ferredoxin, the disulfide bond is converted into two free sulfhydryl groups. Reduced thioredoxin can cleave disulfide bonds in enzymes, activating certain Calvin cycle enzymes and inactivating some degradative enzymes.
[Drawn from 1F9M.pdb.]
Light-driven reactions lead to electron transfer from water to ferredoxin and, eventually, to NADPH. The presence of reduced ferredoxin and NADPH are good signals that conditions are right for biosynthesis. One way in which this information is conveyed to biosynthetic enzymes is by thioredoxin, a 12-kDa protein containing neighboring cysteine residues that cycle between a reduced sulfhydryl and an oxidized disulfide form (Figure 20.14). The reduced form of thioredoxin activates many biosynthetic enzymes, including the chloroplast ATP synthase, by reducing disulfide bridges that control their activity. Reduced thioredoxin also inhibits several degradative enzymes by the same means (Table 20.1). In chloroplasts, oxidized thioredoxin is reduced by ferredoxin in a reaction catalyzed by ferredoxin–thioredoxin reductase. Thus, the activities of the light and dark reactions of photosynthesis are coordinated through electron transfer from reduced ferredoxin to thioredoxin and then to component enzymes containing regulatory disulfide bonds (Figure 20.15). We shall return to thioredoxin when we consider the reduction of ribonucleotides (Section 25.3).
|
|
|
|
|
Carbon fixation in the Calvin cycle |
Fructose 1,6-bisphosphatase |
|
Glyceraldehyde 3-phosphate dehydrogenase |
Calvin cycle, gluconeogenesis, glycolysis |
Sedoheptulose 1,7-bisphosphatase |
|
Glucose 6-phosphate dehydrogenase |
Pentose phosphate pathway |
Phenylalanine ammonia lyase |
|
|
|
|
NADP+-malate dehydrogenase |
|
|
|
|
Table 20.1: Enzymes regulated by thioredoxin
NADPH is a signal molecule that activates two biosynthetic enzymes, phosphoribulokinase and glyceraldehyde 3-phosphate dehydrogenase. In the dark, these enzymes are inhibited by association with an 8.5-kDa protein called CP12, an intrinsically disordered protein (Section 2.6). NADPH disrupts this association by promoting the formation of two disulfide bonds in CP12, leading to the release of the active enzymes.
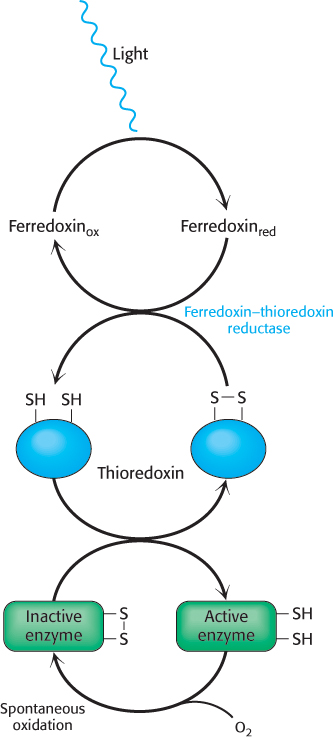
Figure 20.15: Enzyme activation by thioredoxin. Reduced thioredoxin activates certain Calvin cycle enzymes by cleaving regulatory disulfide bonds.
The C4 pathway of tropical plants accelerates photosynthesis by concentrating carbon dioxide
The oxygenase activity of rubisco presents a biochemical challenge to tropical plants because the oxygenase activity increases more rapidly with temperature than does the carboxylase activity. How, then, do plants that grow in hot climates,such as sugar cane, prevent very high rates of wasteful photorespiration? Their solution to this problem is to achieve a high local concentration of CO2 at the site of the Calvin cycle in their photosynthetic cells. The essence of this process, which was elucidated by Marshall Davidson Hatch and C. Roger Slack, is that four-carbon (C4) compounds such as oxaloacetate and malate carry CO2 from mesophyll cells, which are in contact with air, to bundle-sheath cells, which are the major sites of photosynthesis (Figure 20.16). The decarboxylation of the four-carbon compound in a bundle-sheath cell maintains a high concentration of CO2 at the site of the Calvin cycle. The three-carbon product returns to the mesophyll cell for another round of carboxylation.
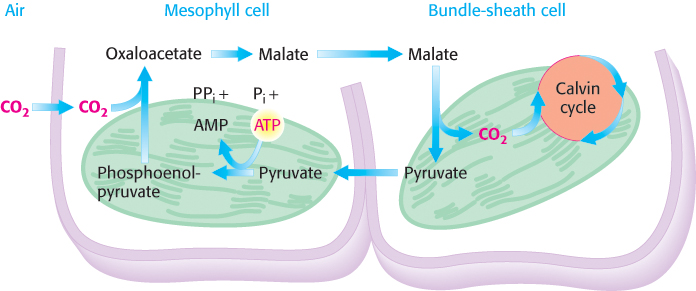
Figure 20.16: C4 pathway. Carbon dioxide is concentrated in bundle-sheath cells by the expenditure of ATP in mesophyll cells.
The C4 pathway for the transport of CO2 starts in a mesophyll cell with the condensation of CO2 and phosphoenolpyruvate to form oxaloacetate in a reaction catalyzed by phosphoenolpyruvate carboxylase. In some species, oxaloacetate is converted into malate by an NADP+-linked malate dehydrogenase. Malate enters the bundle-sheath cell and is oxidatively decarboxylated within the chloroplasts by an NADP+–linked malate dehydrogenase. The released CO2 enters the Calvin cycle in the usual way by condensing with ribulose 1,5-bisphosphate. Pyruvate formed in this decarboxylation reaction returns to the mesophyll cell. Finally, phosphoenolpyruvate is formed from pyruvate by pyruvate-Pi dikinase.
The net reaction of this C4 pathway is
Thus, the energetic equivalent of two ATP molecules is consumed in transporting CO2 to the chloroplasts of the bundle-sheath cells. In essence, this process is active transport: the pumping of CO2 into the bundle-sheath cell is driven by the hydrolysis of one molecule of ATP to one molecule of AMP and two molecules of orthophosphate. The CO2 concentration can be 20-fold as great in the bundle-sheath cells as in the mesophyll cells.
When the C4 pathway and the Calvin cycle operate together, the net reaction is
Note that 30 molecules of ATP are consumed per hexose molecule formed when the C4 pathway delivers CO2 to the Calvin cycle, in contrast with 18 molecules of ATP per hexose molecule in the absence of the C4 pathway. The high concentration of CO2 in the bundle-sheath cells of C4 plants, which is the result of the expenditure of the additional 12 molecules of ATP, is critical for their rapid photosynthetic rate, because CO2 is limiting when light is abundant. A high CO2 concentration also minimizes the energy loss caused by photorespiration.
Tropical plants with a C4 pathway do little photorespiration because the high concentration of CO2 in their bundle-sheath cells accelerates the carboxylase reaction relative to the oxygenase reaction. This effect is especially important at higher temperatures. The geographic distribution of plants having this pathway (C4 plants) and those lacking it (C3 plants) can now be understood in molecular terms. C4 plants have the advantage in a hot environment and under high illumination, which accounts for their prevalence in the tropics. C3 plants, which consume only 18 molecules of ATP per hexose molecule formed in the absence of photorespiration (compared with 30 molecules of ATP for C4 plants), are more efficient at temperatures lower than about 28°C, and so they predominate in temperate environments.
 Rubisco is present in bacteria, eukaryotes, and even archaea, although other photosynthetic components have not been found in archaea. Thus, rubisco emerged early in evolution, when the atmosphere was rich in CO2 and almost devoid of O2. The enzyme was not originally selected to operate in an environment like the present one, which is almost devoid of CO2 and rich in O2. Photorespiration became significant about 600 million years ago, when the CO2 concentration fell to present levels. The C4 pathway is thought to have evolved in response no more than 30 million years ago and possibly as recently as 7 million years ago. It is interesting that none of the enzymes are unique to C4 plants, suggesting that this pathway made use of already existing enzymes.
Rubisco is present in bacteria, eukaryotes, and even archaea, although other photosynthetic components have not been found in archaea. Thus, rubisco emerged early in evolution, when the atmosphere was rich in CO2 and almost devoid of O2. The enzyme was not originally selected to operate in an environment like the present one, which is almost devoid of CO2 and rich in O2. Photorespiration became significant about 600 million years ago, when the CO2 concentration fell to present levels. The C4 pathway is thought to have evolved in response no more than 30 million years ago and possibly as recently as 7 million years ago. It is interesting that none of the enzymes are unique to C4 plants, suggesting that this pathway made use of already existing enzymes.
Crassulacean acid metabolism permits growth in arid ecosystems
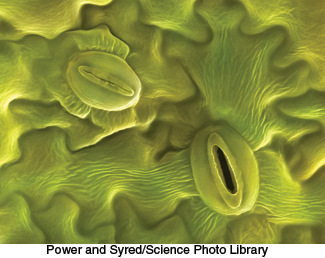
Figure 20.17: Scanning electron micrograph of an open stoma and a closed stoma.
[Power and Syred/Science Photo Library.]
Many plants, including some that grow in hot, dry climates, keep the stomata of their leaves closed in the heat of the day to prevent water loss (Figure 20.17). As a consequence, CO2 cannot be absorbed during the daylight hours, when it is needed for glucose synthesis. Rather, CO2 enters the leaf when the stomata open at the cooler temperatures of night. To store the CO2 until it can be used during the day, such plants make use of an adaptation called crassulacean acid metabolism (CAM), named after the genus Crassulacea (the succulents). Carbon dioxide is fixed by the C4 pathway into malate, which is stored in vacuoles. During the day, malate is decarboxylated and the CO2 becomes available to the Calvin cycle. In contrast with C4 plants, CAM plants separate CO2 accumulation from CO2 utilization temporally rather than spatially.
Although CAM plants do prevent water loss, the use of malate as the sole source of CO2 comes at a metabolic cost. Because the malate storage is limited, CAM plants cannot generate CO2 as rapidly as it can be imported by C3 and C4 plants. Consequently, the growth rate of CAM plants is slower than that of C3 and C4 plants. The saguaro cactus, which can live up to 200 years and reach a height of 60 feet, can take 15 years to grow to only 1 foot in height.


 Thioredoxin. The oxidized form of thioredoxin contains a disulfide bond. When thioredoxin is reduced by reduced ferredoxin, the disulfide bond is converted into two free sulfhydryl groups. Reduced thioredoxin can cleave disulfide bonds in enzymes, activating certain Calvin cycle enzymes and inactivating some degradative enzymes.
Thioredoxin. The oxidized form of thioredoxin contains a disulfide bond. When thioredoxin is reduced by reduced ferredoxin, the disulfide bond is converted into two free sulfhydryl groups. Reduced thioredoxin can cleave disulfide bonds in enzymes, activating certain Calvin cycle enzymes and inactivating some degradative enzymes.


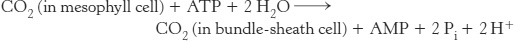
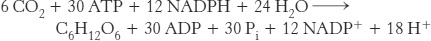
 Rubisco is present in bacteria, eukaryotes, and even archaea, although other photosynthetic components have not been found in archaea. Thus, rubisco emerged early in evolution, when the atmosphere was rich in CO2 and almost devoid of O2. The enzyme was not originally selected to operate in an environment like the present one, which is almost devoid of CO2 and rich in O2. Photorespiration became significant about 600 million years ago, when the CO2 concentration fell to present levels. The C4 pathway is thought to have evolved in response no more than 30 million years ago and possibly as recently as 7 million years ago. It is interesting that none of the enzymes are unique to C4 plants, suggesting that this pathway made use of already existing enzymes.
Rubisco is present in bacteria, eukaryotes, and even archaea, although other photosynthetic components have not been found in archaea. Thus, rubisco emerged early in evolution, when the atmosphere was rich in CO2 and almost devoid of O2. The enzyme was not originally selected to operate in an environment like the present one, which is almost devoid of CO2 and rich in O2. Photorespiration became significant about 600 million years ago, when the CO2 concentration fell to present levels. The C4 pathway is thought to have evolved in response no more than 30 million years ago and possibly as recently as 7 million years ago. It is interesting that none of the enzymes are unique to C4 plants, suggesting that this pathway made use of already existing enzymes.