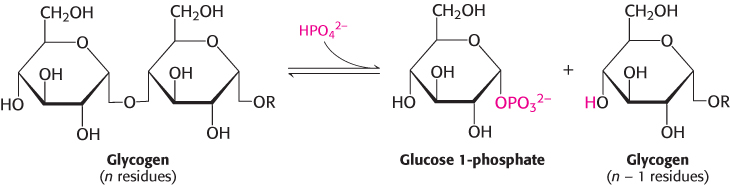
Phosphorylase catalyzes the phosphorolytic cleavage of glycogen to release glucose 1-phosphate
Glycogen phosphorylase, the key enzyme in glycogen breakdown, cleaves its substrate by the addition of orthophosphate (Pi) to yield glucose 1-phosphate. The cleavage of a bond by the addition of orthophosphate is referred to as phosphorolysis.
Phosphorylase catalyzes the sequential removal of glucosyl residues from the nonreducing ends of the glycogen molecule (the ends with a free OH group on carbon 4). Orthophosphate splits the glycosidic linkage between C-1 of the terminal residue and C-4 of the adjacent one. Specifically, it cleaves the bond between the C-1 carbon atom and the glycosidic oxygen atom, and the α configuration at C-1 is retained.
Glucose 1-phosphate released from glycogen can be readily converted into glucose 6-phosphate, an important metabolic intermediate, by the enzyme phosphoglucomutase.
The reaction catalyzed by phosphorylase is readily reversible in vitro. At pH 6.8, the equilibrium ratio of orthophosphate to glucose 1-phosphate is 3.6. The value of ΔG°′ for this reaction is small because a glycosidic bond is replaced by a phosphoryl ester bond that has a nearly equal transfer potential. However, phosphorolysis proceeds far in the direction of glycogen breakdown in vivo because the [Pi]/[glucose 1-phosphate] ratio is usually greater than 100, substantially favoring phosphorolysis. We see here an example of how the cell can alter the free-energy change to favor a reaction’s occurrence by altering the ratio of substrate and product.
The phosphorolytic cleavage of glycogen is energetically advantageous because the released sugar is already phosphorylated. In contrast, a hydrolytic cleavage would yield glucose, which would then have to be phosphorylated at the expense of a molecule of ATP to enter the glycolytic pathway. An additional advantage of phosphorolytic cleavage for muscle cells is that no transporters exist for glucose 1-phosphate, which is negatively charged under physiological conditions, and so it cannot be transported or diffuse out of the cell.
Mechanism: Pyridoxal phosphate participates in the phosphorolytic cleavage of glycogen
The special challenge faced by phosphorylase is to cleave glycogen phosphorolytically rather than hydrolytically to save the ATP required to phosphorylate free glucose. Thus, water must be excluded from the active site. Phosphorylase is a dimer of two identical 97-kDa subunits. Each subunit is compactly folded into an amino-terminal domain (480 residues) containing a glycogen-binding site and a carboxyl-terminal domain (360 residues; Figure 21.5). The catalytic site in each subunit is located in a deep crevice formed by residues from both domains. Substrates bind synergistically, causing the crevice to narrow, thereby excluding water. What is the mechanistic basis of the phosphorolytic cleavage of glycogen?
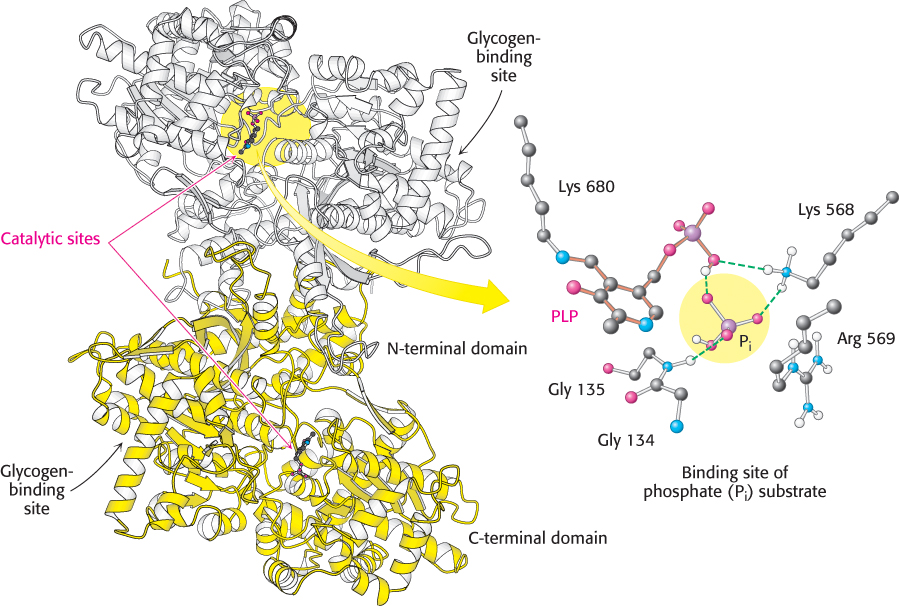
Figure 21.5:  Structure of glycogen phosphorylase. This enzyme forms a homodimer: one subunit is shown in white and the other in yellow. Each catalytic site includes a pyridoxal phosphate (PLP) group, linked to lysine 680 of the enzyme. The binding site for the phosphate (Pi) substrate is shown. Notice that the catalytic site lies between the C-terminal domain and the glycogen-binding site. A narrow crevice, which binds four or five glucose units of glycogen, connects the two sites. The separation of the sites allows the catalytic site to phosphorolyze several glucose units before the enzyme must rebind the glycogen substrate.
Structure of glycogen phosphorylase. This enzyme forms a homodimer: one subunit is shown in white and the other in yellow. Each catalytic site includes a pyridoxal phosphate (PLP) group, linked to lysine 680 of the enzyme. The binding site for the phosphate (Pi) substrate is shown. Notice that the catalytic site lies between the C-terminal domain and the glycogen-binding site. A narrow crevice, which binds four or five glucose units of glycogen, connects the two sites. The separation of the sites allows the catalytic site to phosphorolyze several glucose units before the enzyme must rebind the glycogen substrate.
[Drawn from 1NOI.pdb.]
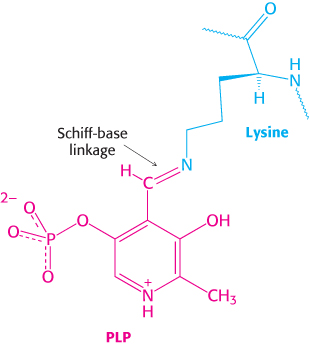
Figure 21.6: PLP–Schiff-base linkage. A pyridoxal phosphate (PLP) group (red) forms a Schiff base with a lysine residue (blue) at the active site of phosphorylase, where it functions as a general acid–base catalyst.
Several clues suggest a mechanism. First, both the glycogen substrate and the glucose 1-phosphate product have an α configuration at C-1. A direct attack by phosphate on C-1 of a sugar would invert the configuration at this carbon atom because the reaction would proceed through a pentacovalent transition state. The fact that the glucose 1-phosphate formed has an α rather than a β configuration suggests that an even number of steps (most simply, two) is required. A likely explanation for these results is that a carbonium ion intermediate is formed from the glucose residue.
A second clue to the catalytic mechanism of phosphorylase is its requirement for the coenzyme pyridoxal phosphate (PLP), a derivative of pyridoxine (vitamin B6, Section 15.4). The aldehyde group of this coenzyme forms a Schiff-base linkage with a specific lysine side chain of the enzyme (Figure 21.6). Structural studies indicate that the reacting orthophosphate group takes a position between the 5′-phosphate group of PLP and the glycogen substrate (Figure 21.7). The 5′-phosphate group of PLP acts in tandem with orthophosphate by serving as a proton donor and then as a proton acceptor (i.e., as a general acid–base catalyst). Orthophosphate (in the HPO42− form) donates a proton to the oxygen atom attached to carbon 4 of the departing glycogen chain and simultaneously acquires a proton from PLP. The carbonium ion intermediate formed in this step is then attacked by orthophosphate to form α-glucose 1-phosphate, with the concomitant return of a proton to pyridoxal phosphate. The special role of pyridoxal phosphate in the reaction is necessary because water is excluded from the active site.
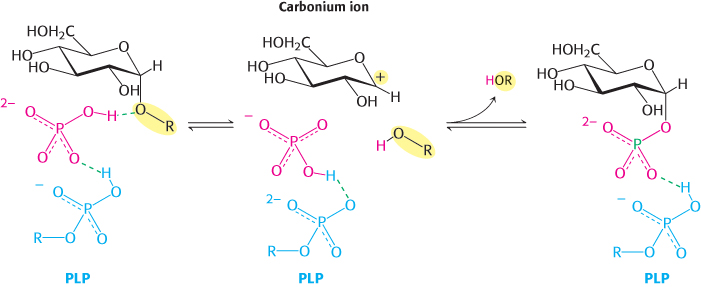
Figure 21.7: Phosphorylase mechanism. A bound HPO42−group (red) favors the cleavage of the glycosidic bond by donating a proton to the C-4 oxygen of the departing glycosyl group (black). This reaction results in the formation of a carbonium ion and is favored by the transfer of a proton from the protonated phosphate group of the bound pyridoxal phosphate (PLP) group (blue). The carbonium ion and the orthophosphate combine to form glucose 1-phosphate.
A Schiff base, also called an imine, is a compound containing a carbon–nitrogen double bond with the nitrogen atom bonded to an organic compound, not a hydrogen atom. A Schiff base is formed by the reaction of a primary amine with an aldehyde or ketone.
The glycogen-binding site is 30 Å away from the catalytic site (Figure 21.5), but it is connected to the catalytic site by a narrow crevice able to accommodate four or five glucose units. The large separation between the binding site and the catalytic site enables the enzyme to phosphorolyze many residues without having to dissociate and reassociate after each catalytic cycle. An enzyme that can catalyze many reactions without having to dissociate and reassociate after each catalytic step is said to be processive—a property of enzymes that synthesize and degrade large polymers. We will see such enzymes again when we consider DNA and RNA synthesis.
A debranching enzyme also is needed for the breakdown of glycogen
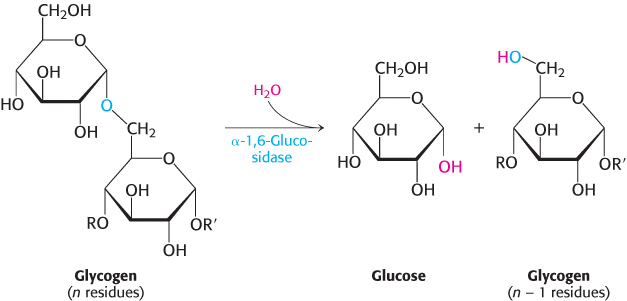
Glycogen phosphorylase acting alone degrades glycogen to a limited extent. The enzyme can break α-1,4-glycosidic bonds on glycogen branches but soon encounters an obstacle. The α-1,6-glycosidic bonds at the branch points are not susceptible to cleavage by phosphorylase. Indeed, phosphorylase stops cleaving α-1,4 linkages when it reaches a terminal residue four residues away from a branch point. Because about 1 in 12 residues is branched, cleavage by the phosphorylase alone would come to a halt after the release of eight glucose molecules per branch.
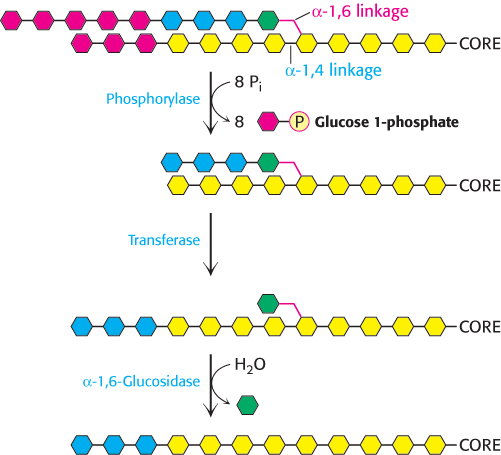
Figure 21.8: Glycogen remodeling. First, α-1,4-glycosidic bonds on each branch are cleaved by phosphorylase, leaving four residues along each branch. The transferase shifts a block of three glucosyl residues from one outer branch to the other. In this reaction, the α-1,4-glycosidic link between the blue and the green residues is broken and a new α-1,4 link between the blue and the yellow residues is formed. The green residue is then removed by α-1,6-glucosidase, leaving a linear chain with all α-1,4 linkages, suitable for further cleavage by phosphorylase.
How can the remainder of the glycogen molecule be mobilized for use as a fuel? Two additional enzymes, a transferase and α-1,6-glucosidase, remodel the glycogen for continued degradation by the phosphorylase (Figure 21.8). The transferase shifts a block of three glucosyl residues from one outer branch to another. This transfer exposes a single glucose residue joined by an α-1,6-glycosidic linkage. α-1,6-Glucosidase, also known as the debranching enzyme, hydrolyzes the α-1,6-glycosidic bond.
A free glucose molecule is released and then phosphorylated by the glycolytic enzyme hexokinase. Thus, the transferase and α-1,6-glucosidase convert the branched structure into a linear one, which paves the way for further cleavage by phosphorylase. In eukaryotes, the transferase and the α-1,6-glucosidase activities are present in a single 160-kDa polypeptide chain, providing yet another example of a bifunctional enzyme (Figure 16.29).
Phosphoglucomutase converts glucose 1-phosphate into glucose 6-phosphate
Glucose 1-phosphate formed in the phosphorolytic cleavage of glycogen must be converted into glucose 6-phosphate to enter the metabolic mainstream. This shift of a phosphoryl group is catalyzed by phosphoglucomutase. Recall that this enzyme is also used in galactose metabolism (Section 16.1). To effect this shift, the enzyme exchanges a phosphoryl group with the substrate (Figure 21.9). The catalytic site of an active mutase molecule contains a phosphorylated serine residue. The phosphoryl group is transferred from the serine residue to the C-6 hydroxyl group of glucose 1-phosphate to form glucose 1,6-bisphosphate. The C-1 phosphoryl group of this intermediate is then shuttled to the same serine residue, resulting in the formation of glucose 6-phosphate and the regeneration of the phosphoenzyme.
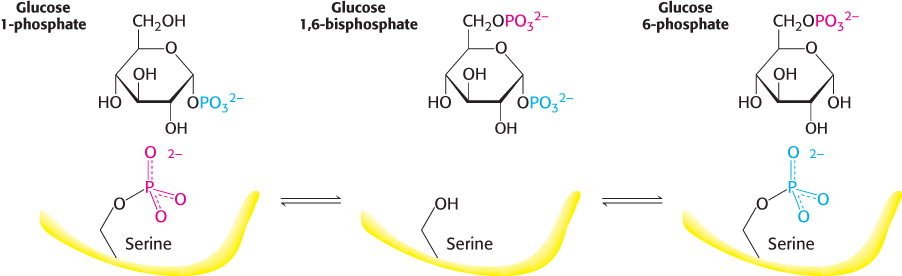
Figure 21.9: Reaction catalyzed by phosphoglucomutase. A phosphoryl group is transferred from the enzyme to the substrate, and a different phosphoryl group is transferred back to restore the enzyme to its initial state.
These reactions are like those of phosphoglycerate mutase, a glycolytic enzyme (Section 16.1). The role of glucose 1,6-bisphosphate in the interconversion of the phosphoglucoses is like that of 2,3-bisphosphoglycerate (2,3-BPG) in the interconversion of 2-phosphoglycerate and 3-phosphoglycerate in glycolysis. A phosphoenzyme intermediate participates in both reactions.
The liver contains glucose 6-phosphatase, a hydrolytic enzyme absent from muscle
A major function of the liver is to maintain a nearly constant level of glucose in the blood. The liver releases glucose into the blood between meals and during muscular activity. The released glucose is taken up primarily by the brain, skeletal muscle, and red blood cells. In contrast with unmodified glucose, however, the phosphorylated glucose produced by glycogen breakdown is not transported out of cells. The liver contains a hydrolytic enzyme, glucose 6-phosphatase, that enables glucose to leave that organ. This enzyme cleaves the phosphoryl group to form free glucose and orthophosphate. This glucose 6-phosphatase is the same enzyme that releases free glucose at the conclusion of gluconeogenesis. It is located on the lumenal side of the smooth endoplasmic reticulum membrane. Recall that glucose 6-phosphate is transported into the endoplasmic reticulum; glucose and orthophosphate formed by hydrolysis are then shuttled back into the cytoplasm (Section 16.1).
Glucose 6-phosphate + H2O → glucose + Pi
Glucose 6-phosphatase is absent from most other tissues. Muscle tissues retain glucose 6-phosphate for the generation of ATP. In contrast, glucose is not a major fuel for the liver.
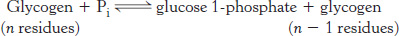

 Structure of glycogen phosphorylase. This enzyme forms a homodimer: one subunit is shown in white and the other in yellow. Each catalytic site includes a pyridoxal phosphate (PLP) group, linked to lysine 680 of the enzyme. The binding site for the phosphate (Pi) substrate is shown. Notice that the catalytic site lies between the C-
Structure of glycogen phosphorylase. This enzyme forms a homodimer: one subunit is shown in white and the other in yellow. Each catalytic site includes a pyridoxal phosphate (PLP) group, linked to lysine 680 of the enzyme. The binding site for the phosphate (Pi) substrate is shown. Notice that the catalytic site lies between the C-