23.1 Proteins Are Degraded to Amino Acids
|
Histidine |
|
Isoleucine |
|
Leucine |
|
Lysine |
|
Methionine |
|
Phenylalanine |
|
Threonine |
|
Tryptophan |
|
Valine |
Dietary protein is a vital source of amino acids. Especially important dietary proteins are those containing the essential amino acids—
The digestion of dietary proteins begins in the stomach and is completed in the intestine
 Protein digestion begins in the stomach, where the acidic environment denatures proteins into random coils. Denatured proteins are more accessible as substrates for proteolysis than are native proteins. The primary proteolytic enzyme of the stomach is pepsin, a nonspecific protease that, remarkably, is maximally active at pH 2. Thus, pepsin can function in the highly acidic environment of the stomach that disables other proteins.
Protein digestion begins in the stomach, where the acidic environment denatures proteins into random coils. Denatured proteins are more accessible as substrates for proteolysis than are native proteins. The primary proteolytic enzyme of the stomach is pepsin, a nonspecific protease that, remarkably, is maximally active at pH 2. Thus, pepsin can function in the highly acidic environment of the stomach that disables other proteins.
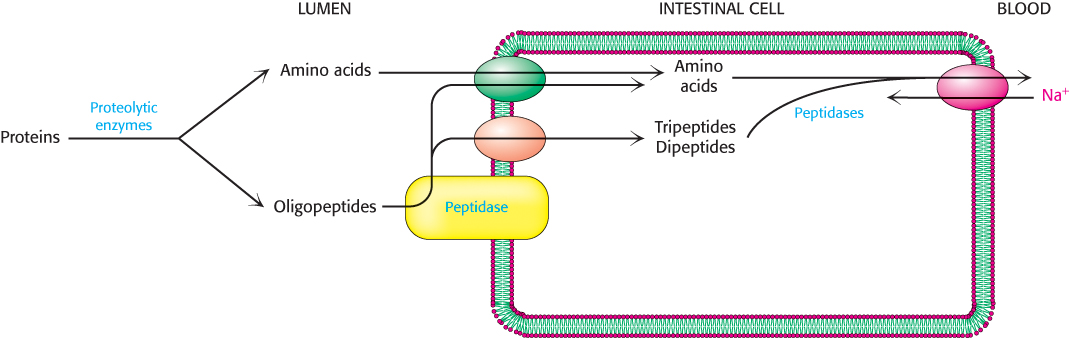
The partly digested proteins then move from the acidic environment of the stomach to the beginning of the small intestine. The low pH of the food as well as the polypeptide products of pepsin digestion stimulate the release of hormones that promote the secretion from the pancreas of sodium bicarbonate (NaHCO3), which neutralizes the pH of the food, and a variety of pancreatic proteolytic enzymes. Recall that these enzymes are secreted as inactive zymogens that are then converted into active enzymes (Sections 9.1 and 10.4). The battery of enzymes displays a wide array of specificity, and so the substrates are degraded into free amino acids as well as di-
At least seven different transporters exist, each specific to a different group of amino acids. A number of inherited disorders result from mutations in these transporters. For example, Hartnup disease, a rare disorder characterized by rashes, ataxia (lack of muscle control), delayed mental development, and diarrhea, results from a defect in the transporter for tryptophan and other nonpolar amino acids. The absorbed amino acids are subsequently released into the blood by a number of Na+–amino acid antiporters for use by other tissues (Figure 23.1).
Cellular proteins are degraded at different rates
Protein turnover —the degradation and resynthesis of proteins—
683
The half-