Chapter Introduction
The Biosynthesis of Amino Acids
713
OUTLINE
Nitrogen Fixation: Microorganisms Use ATP and a Powerful Reductant to Reduce Atmospheric Nitrogen to Ammonia
Amino Acids Are Made from Intermediates of the Citric Acid Cycle and Other Major Pathways
Feedback Inhibition Regulates Amino Acid Biosynthesis
Amino Acids Are Precursors of Many Biomolecules
The assembly of biological molecules, including proteins and nucleic acids, requires the generation of appropriate starting materials. We have already considered the assembly of carbohydrates in discussions of the Calvin cycle and the pentose phosphate pathway (Chapter 20). The present chapter and the next two examine the assembly of the other important building blocks—
The pathways for the biosynthesis of these molecules are extremely ancient, going back to the last common ancestor of all living things. Indeed, these pathways probably predate many of the pathways of energy transduction discussed in Part II and may have provided key selective advantages in early evolution. Many of the intermediates in energy-
Anabolism
Biosynthetic processes.
Catabolism
Degradative processes.
Derived from from the the Greek ana, “up” kata, “down” ballein “to throw.”
We begin our consideration of biosynthesis with amino acids—
714
Amino acid synthesis requires solutions to three key biochemical problems
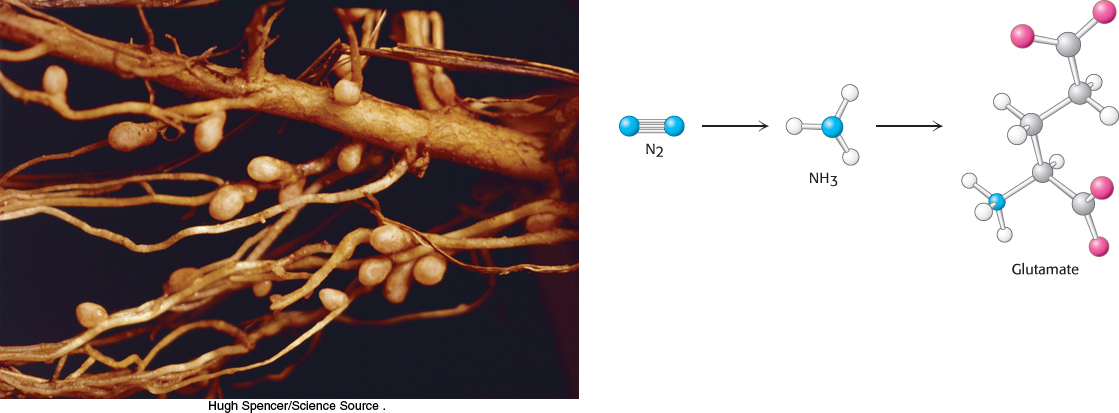
Nitrogen is an essential component of amino acids. Earth has an abundant supply of nitrogen, but it is primarily in the form of atmospheric nitrogen gas (N2), a remarkably inert molecule. Thus, a fundamental problem for biological systems is to obtain nitrogen in a more usable form. This problem is solved by certain microorganisms capable of reducing the inert N ≡ N molecule of nitrogen gas to two molecules of ammonia in one of the most amazing reactions in biochemistry. Nitrogen in the form of ammonia is the source of nitrogen for all the amino acids. The carbon backbones come from the glycolytic pathway, the pentose phosphate pathway, or the citric acid cycle.
In amino acid production, we encounter an important problem in biosynthesis—
Biosynthetic pathways are often highly regulated such that building blocks are synthesized only when supplies are low. Very often, a high concentration of the final product of a pathway inhibits the activity of allosteric enzymes that function early in the pathway to control the committed step. These enzymes are similar in functional properties to aspartate transcar-