Chapter Introduction
Nucleotide Biosynthesis
743
OUTLINE
The Pyrimidine Ring Is Assembled de Novo or Recovered by Salvage Pathways
Purine Bases Can Be Synthesized de Novo or Recycled by Salvage Pathways
Deoxyribonucleotides Are Synthesized by the Reduction of Ribonucleotides Through a Radical Mechanism
Key Steps in Nucleotide Biosynthesis Are Regulated by Feedback Inhibition
Disruptions in Nucleotide Metabolism Can Cause Pathological Conditions
Nucleotides are key biomolecules required for a variety of life processes. First, nucleotides are the activated precursors of nucleic acids, necessary for the replication of the genome and the transcription of the genetic information into RNA. Second, an adenine nucleotide, ATP, is the universal currency of energy. A guanine nucleotide, GTP, also serves as an energy source for a more select group of biological processes. Third, nucleotide derivatives such as UDP-
In this chapter, we continue along the path begun in Chapter 24, which described the incorporation of nitrogen into amino acids from inorganic sources such as nitrogen gas. The amino acids glycine and aspartate are the scaffolds on which the ring systems present in nucleotides are assembled. Furthermore, aspartate and the side chain of glutamine serve as sources of NH2 groups in the formation of nucleotides.
744
Nucleotide biosynthetic pathways are tremendously important as intervention points for therapeutic agents. Many of the most widely used drugs in the treatment of cancer block steps in nucleotide biosynthesis, particularly steps in the synthesis of DNA precursors.
Nucleotides can be synthesized by de novo or salvage pathways
The pathways for the biosynthesis of nucleotides fall into two classes: de novo pathways and salvage pathways (Figure 25.1). In de novo (from scratch) pathways, the nucleotide bases are assembled from simpler compounds. The framework for a pyrimidine base is assembled first and then attached to ribose. In contrast, the framework for a purine base is synthesized piece by piece directly onto a ribose-
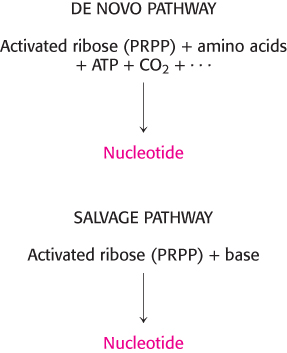
De novo pathways lead to the synthesis of ribonucleotides. However, DNA is built from deoxyribonucleotides. Consistent with the notion that RNA preceded DNA in the course of evolution, all deoxyribonucleotides are synthesized from the corresponding ribonucleotides. The deoxyribose sugar is generated by the reduction of ribose within a fully formed nucleotide. Furthermore, the methyl group that distinguishes the thymine of DNA from the uracil of RNA is added at the last step in the pathway.
The nomenclature of nucleotides and their constituent units was presented in Chapter 4. Recall that a nucleoside is a purine or pyrimidine base linked to a sugar and that a nucleotide is a phosphate ester of a nucleoside. The names of the major bases of RNA and DNA, and of their nucleoside and nucleotide derivatives, are given in Table 25.1.
|
RNA |
|
|
|---|---|---|
|
Base |
Ribonucleoside |
Ribonucleotide (5′-monophosphate) |
|
Adenine (A) |
Adenosine |
Adenylate (AMP) |
|
Guanine (G) |
Guanosine |
Guanylate (GMP) |
|
Uracil (U) |
Uridine |
Uridylate (UMP) |
|
Cytosine (C) |
Cytidine |
Cytidylate (CMP) |
|
DNA |
|
|
|
Base |
Deoxyribonucleoside |
Deoxyribonucleotide (5′-monophosphate) |
|
Adenine (A) |
Deoxyadenosine |
Deoxyadenylate (dAMP) |
|
Guanine (G) |
Deoxyguanosine |
Deoxyguanylate (dGMP) |
|
Thymine (T) |
Thymidine |
Thymidylate (TMP) |
|
Cytosine (C) |
Deoxycytidine |
Deoxycytidylate (dCMP) |