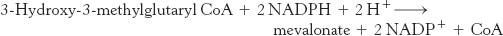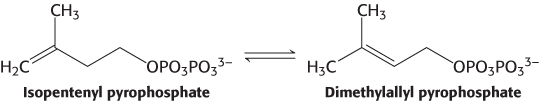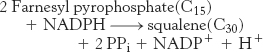26.2 Cholesterol Is Synthesized from Acetyl Coenzyme A in Three Stages
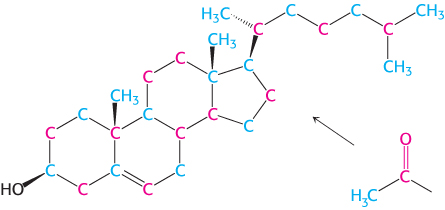
Figure 26.7: Labeling of cholesterol. Isotope-labeling experiments reveal the source of carbon atoms in cholesterol synthesized from acetate labeled in its methyl group (blue) or carboxylate atom (red).
We now turn our attention to the synthesis of the fundamental lipid cholesterol. This steroid modulates the fluidity of animal cell membranes (Section 12.5) and is the precursor of steroid hormones such as progesterone, testosterone, estradiol, and cortisol. All 27 carbon atoms of cholesterol are derived from acetyl CoA in a three-stage synthetic process (Figure 26.7).
1. Stage one is the synthesis of isopentenyl pyrophosphate, an activated isoprene unit that is the key building block of cholesterol.
2. Stage two is the condensation of six molecules of isopentenyl pyrophosphate to form squalene.
3. In stage three, squalene cyclizes and the tetracyclic product is subsequently converted into cholesterol.
The first stage takes place in the cytoplasm, and the next two in the endoplasmic reticulum.
The synthesis of mevalonate, which is activated as isopentenyl pyrophosphate, initiates the synthesis of cholesterol
Cholesterol
“Cholesterol is the most highly decorated small molecule in biology. Thirteen Nobel Prizes have been awarded to scientists who devoted major parts of their careers to cholesterol. Ever since it was isolated from gallstones in 1784, cholesterol has exerted an almost hypnotic fascination for scientists from the most diverse areas of science and medicine…. Cholesterol is a Janus-faced molecule. The very property that makes it useful in cell membranes, namely its absolute insolubility in water, also makes it lethal.”
—Michael Brown and Joseph Goldstein, on the occasion of their receipt of a Nobel Prize for elucidating the control of blood levels of cholesterol.
Nobel Lectures (1985) © The Nobel Foundation, 1985
The first stage in the synthesis of cholesterol is the formation of isopentenyl pyrophosphate from acetyl CoA. This set of reactions starts with the formation of 3-hydroxy-3-methylglutaryl CoA (HMG CoA) from acetyl CoA and acetoacetyl CoA. This intermediate is reduced to mevalonate for the synthesis of cholesterol (Figure 26.8). Recall that, alternatively, 3-hydroxy-3-methylglutaryl CoA may be generated in the mitochondria and processed to form ketone bodies, which are subsequently secreted to provide fuel for other tissues, notably the brain under starvation conditions (Section 22.3).
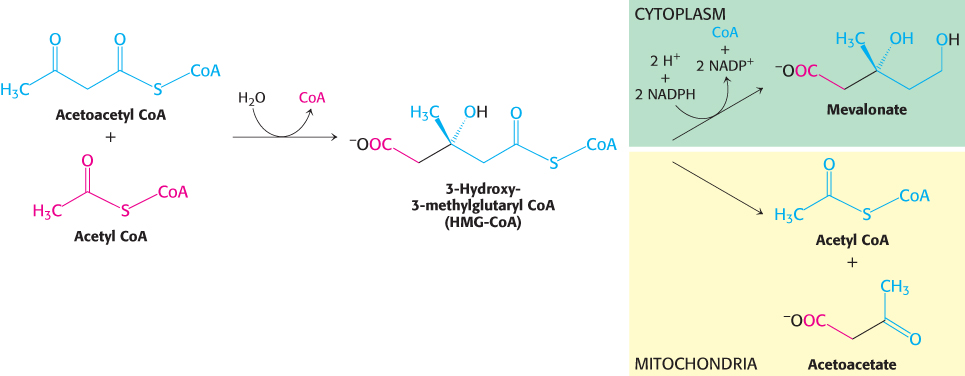
Figure 26.8: Fates of 3-hydroxy-3-methylglutaryl CoA. In the cytoplasm, HMG-CoA is converted into mevalonate. In mitochondria, it is converted into acetyl CoA and acetoacetate.
The synthesis of mevalonate is the committed step in cholesterol formation. The enzyme catalyzing this irreversible step, 3-hydroxy-3-methylglutaryl CoA reductase (HMG-CoA reductase), is an important control site in cholesterol biosynthesis, as will be discussed shortly.
HMG-CoA reductase is an integral membrane protein in the endoplasmic reticulum.
Mevalonate is converted into 3-isopentenyl pyrophosphate in three consecutive reactions requiring ATP (Figure 26.9). In the last step, the release of CO2 yields isopentenyl pyrophosphate, an activated isoprene unit that is a key building block for many important biomolecules throughout the kingdoms of life.
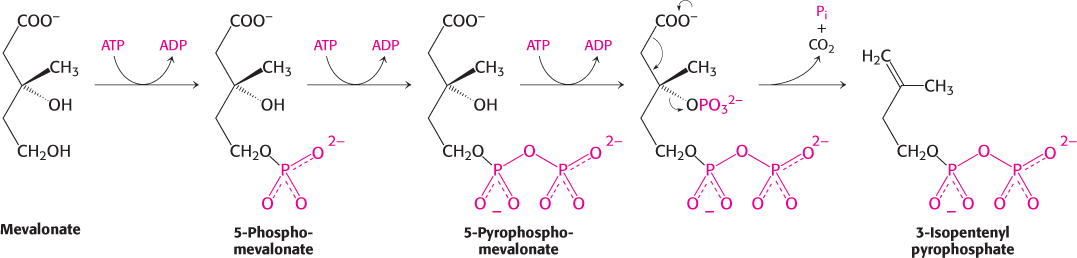
Figure 26.9: Synthesis of isopentenyl pyrophosphate. This activated intermediate is formed from mevalonate in three steps requiring ATP, followed by a decarboxylation.
Squalene (C30) is synthesized from six molecules of isopentenyl pyrophosphate (C5)
Squalene is synthesized from isopentenyl pyrophosphate by the reaction sequence
This stage in the synthesis of cholesterol starts with the isomerization of isopentenyl pyrophosphate to dimethylallyl pyrophosphate.
These two isomeric C5 units (one of each type) condense to form a C10 compound: isopentenyl pyrophosphate attacks an allylic carbocation formed from dimethylallyl pyrophosphate to yield geranyl pyrophosphate (Figure 26.10). The same kind of reaction takes place again: geranyl pyrophosphate is converted into an allylic carbonium ion, which is attacked by isopentenyl pyrophosphate. The resulting C15 compound is called farnesyl pyrophosphate. The same enzyme, geranyl transferase, catalyzes each of these condensations.
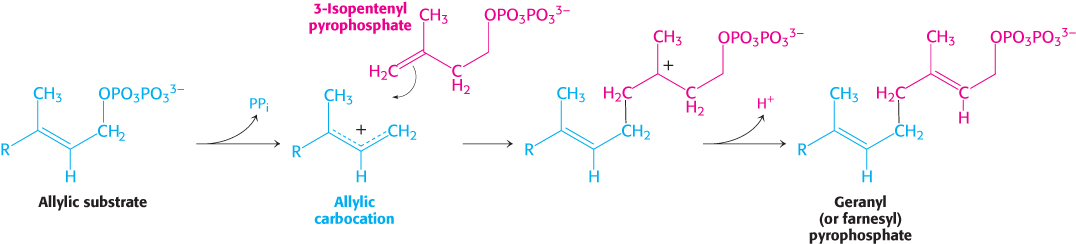
Figure 26.10: Condensation mechanism in cholesterol synthesis. The isomeric C5 units dimethylallyl pyrophosphate and isopentenyl pyrophosphate join to form geranyl pyrophosphate. The same mechanism is used to add an additional isopentenyl pyrophosphate to form farnesyl pyrophosphate.
The last step in the synthesis of squalene is a reductive tail-to-tail condensation of two molecules of farnesyl pyrophosphate catalyzed by the endoplasmic reticulum enzyme squalene synthase.
The reactions leading from C5 units to squalene, a C30 isoprenoid, are summarized in Figure 26.11.
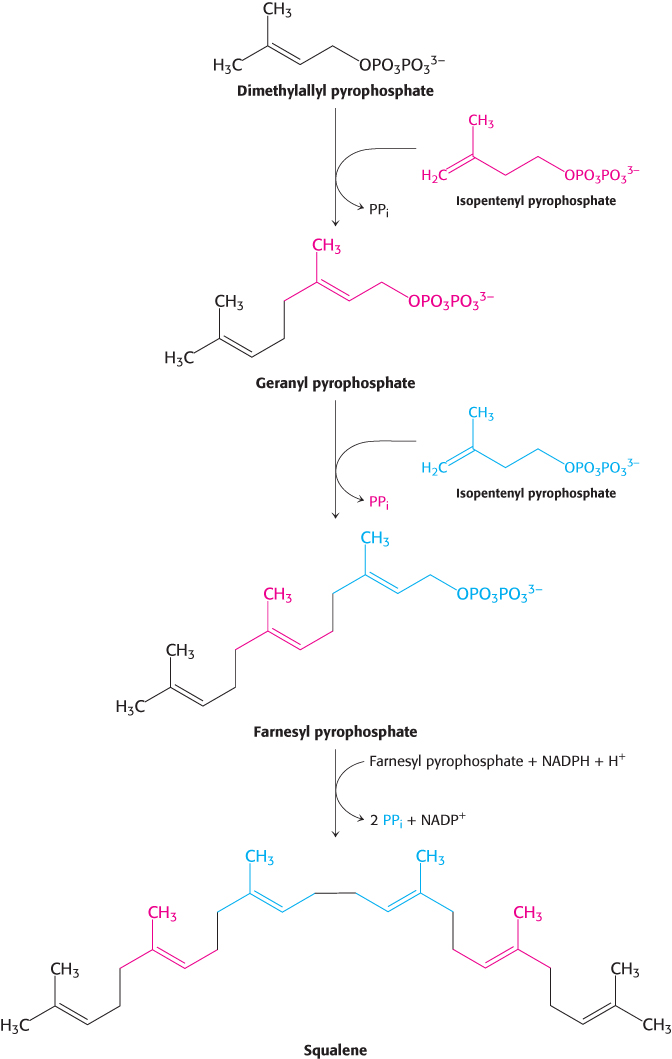
Figure 26.11: Squalene synthesis. One molecule of dimethylallyl pyrophosphate and two molecules of isopentenyl pyrophosphate condense to form farnesyl pyrophosphate. The tail-to-tail coupling of two molecules of farnesyl pyrophosphate yields squalene.
Squalene cyclizes to form cholesterol
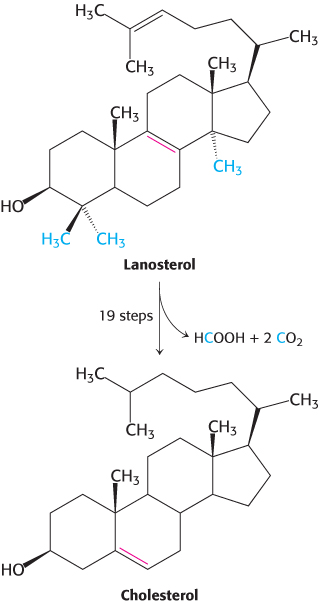
Figure 26.13: Cholesterol formation. Lanosterol is converted into cholesterol in a complex process.
The final stage of cholesterol biosynthesis starts with the cyclization of squalene (Figure 26.12). Squalene is first activated by conversion into squalene epoxide (2,3-oxidosqualene) in a reaction that uses O2 and NADPH. Squalene epoxide is then cyclized to lanosterol by oxidosqualene cyclase. The enzyme holds squalene epoxide in an appropriate conformation and initiates the reaction by protonating the epoxide oxygen. The carbocation formed spontaneously rearranges to produce lanosterol. Lanosterol is converted into cholesterol in a multistep process by the removal of three methyl groups, the reduction of one double bond by NADPH, and the migration of the other double bond (Figure 26.13).
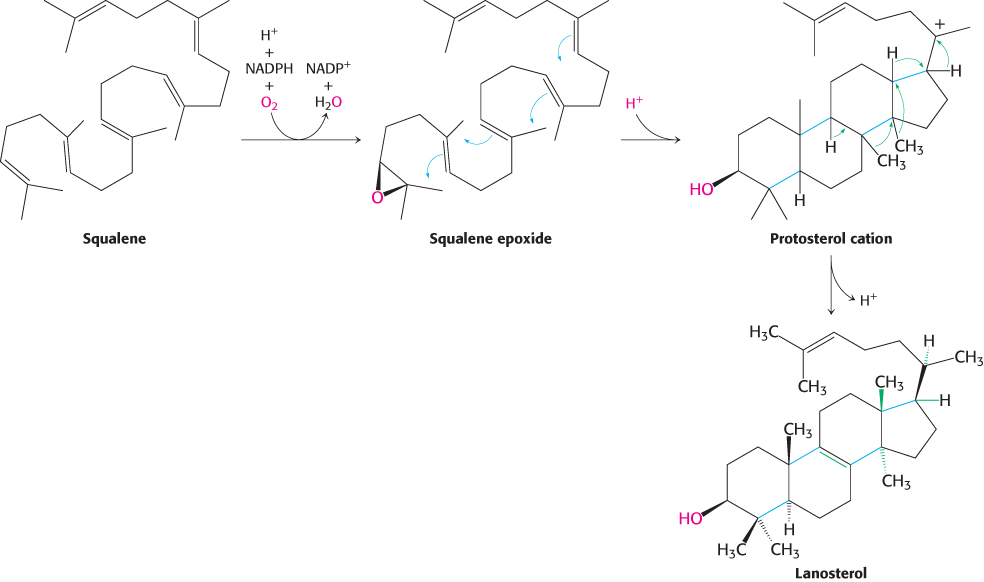
Figure 26.12: Squalene cyclization. The formation of the steroid nucleus from squalene begins with the formation of squalene epoxide. This intermediate is protonated to form a carbocation that cyclizes to form a tetracyclic structure, which rearranges to form lanosterol.