26.4 Important Derivatives of Cholesterol Include Bile Salts and Steroid Hormones
Although cholesterol is well known in its own right as a contributor to the development of heart disease, metabolites of cholesterol—
Bile salts. Bile salts are polar derivatives of cholesterol. These compounds are highly effective detergents because they contain both polar and nonpolar regions. Bile salts are synthesized in the liver, stored and concentrated in the gallbladder, and then released into the small intestine. Bile salts, the major constituent of bile, solubilize dietary lipids. Solubilization increases the effective surface area of lipids with two consequences: (1) more surface area is exposed to the digestive action of lipases and (2) lipids are more readily absorbed by the intestine. Bile salts are also the major breakdown products of cholesterol. The bile salts glycocholate, the primary bile salt, and taurocholate are shown in Figure 26.24.
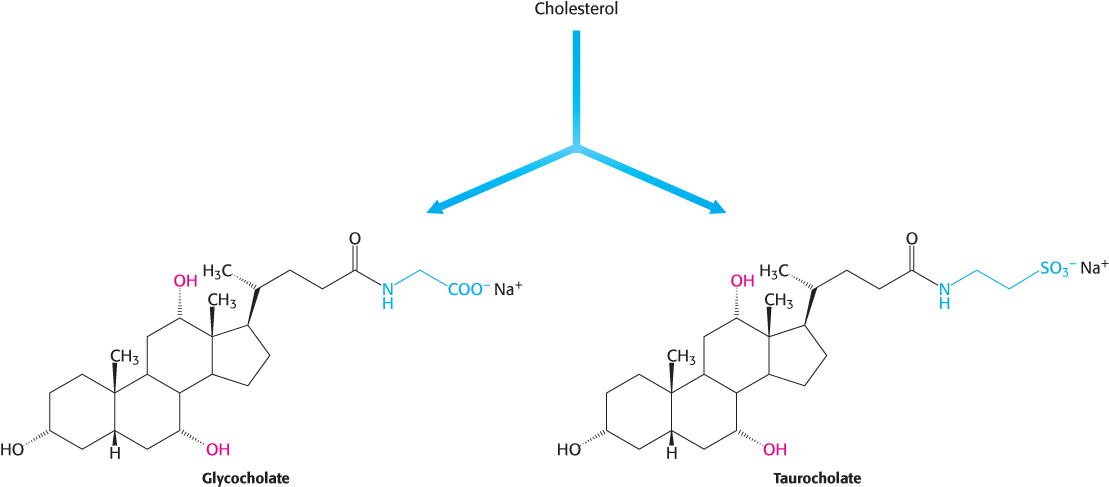
789
 In addition to bile salts, bile is composed of cholesterol, phospholipids, and the breakdown products of heme, bilirubin, and biliverdin (Section 24.4). If too much cholesterol is present in the bile, it will precipitate to form gallbladder stones (cholelithiasis). These stones may block bile secretion and inflame the gallbladder, a condition called cholecystitis. Symptoms include pain in the upper right abdomen, especially after a fatty meal, and nausea. If need be, the gall bladder is removed, and bile flows from the liver through the bile duct directly into the intestine.
In addition to bile salts, bile is composed of cholesterol, phospholipids, and the breakdown products of heme, bilirubin, and biliverdin (Section 24.4). If too much cholesterol is present in the bile, it will precipitate to form gallbladder stones (cholelithiasis). These stones may block bile secretion and inflame the gallbladder, a condition called cholecystitis. Symptoms include pain in the upper right abdomen, especially after a fatty meal, and nausea. If need be, the gall bladder is removed, and bile flows from the liver through the bile duct directly into the intestine.
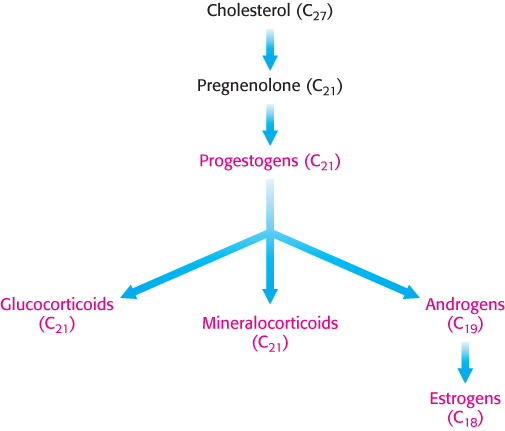
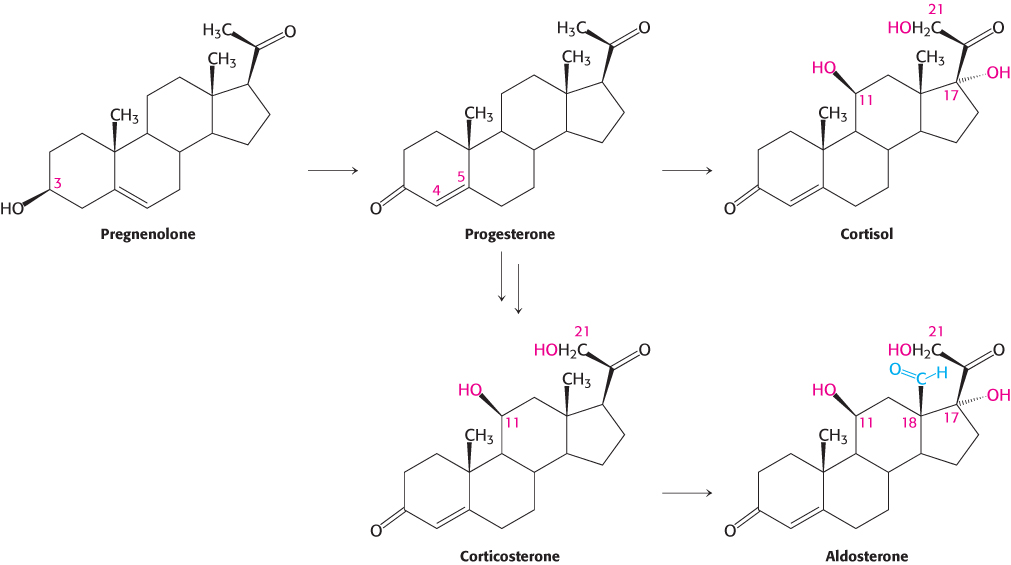
Steroid hormones. Cholesterol is the precursor of the five major classes of steroid hormones: progestagens, glucocorticoids, mineralocorticoids, androgens, and estrogens (Figure 26.25). These hormones are powerful signal molecules that regulate a host of organismal functions. Progesterone, a progestogen, prepares the lining of the uterus for the implantation of an ovum. Progesterone is also essential for the maintenance of pregnancy by preventing premature uterine contractions. Androgens (such as testosterone) are responsible for the development of male secondary sex characteristics, whereas estrogens (such as estradiol) are required for the development of female secondary sex characteristics. Estrogens, along with progesterone, also participate in the ovarian cycle. Glucocorticoids (such as cortisol) promote gluconeogenesis and glycogen synthesis, enhance the degradation of fat and protein, and inhibit the inflammatory response. They enable animals to respond to stress; indeed, the absence of glucocorticoids can be fatal. Mineralocorticoids (primarily aldosterone) act on the distal tubules of the kidney to increase the reabsorption of Na+ and the excretion of K+ and H+, which leads to an increase in blood volume and blood pressure. The major sites of synthesis of these classes of hormones are the corpus luteum, for progestogens; the testes, for androgens; the ovaries, for estrogens; and the adrenal cortex, for glucocorticoids and mineralocorticoids.
Steroid hormones bind to and activate receptor molecules that serve as transcription factors to regulate gene expression (Section 32.2). These small similar molecules are able to have greatly differing effects because the slight structural differences among them allow interactions with specific receptor molecules.
790
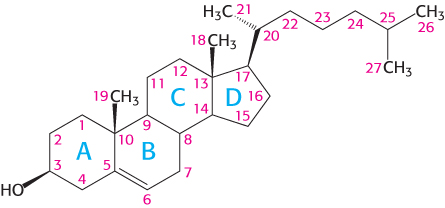
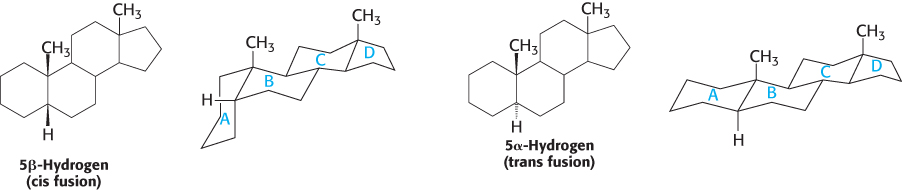
Letters identify the steroid rings and numbers identify the carbon atoms
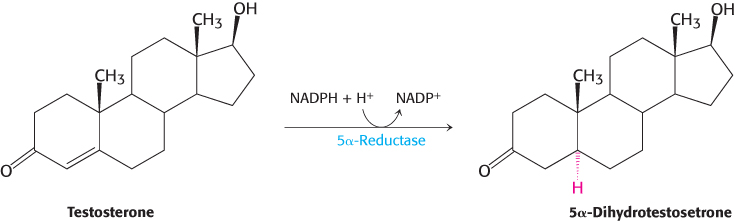
Carbon atoms in steroids are numbered, as shown for cholesterol in Figure 26.26. The rings in steroids are denoted by the letters A, B, C, and D. Cholesterol contains two angular methyl groups: the C-
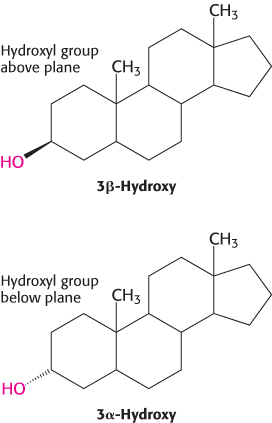
If a hydrogen atom is attached to C-
Steroids are hydroxylated by cytochrome P450 monooxygenases that use NADPH and O2
The addition of OH groups plays an important role in the synthesis of cholesterol from squalene and in the conversion of cholesterol into steroid hormones and bile salts. All these hydroxylations require NADPH and O2. The oxygen atom of the incorporated hydroxyl group comes from O2 rather than from H2O. Whereas one oxygen atom of the O2 molecule goes into the substrate, the other is reduced to water. The enzymes catalyzing these reactions are called monooxygenases (or mixed-
RH + O2 + NADPH + H+ → ROH + H2O + NADP+
Hydroxylation requires the activation of oxygen. In the synthesis of steroid hormones and bile salts, activation is accomplished by members of the cytochrome P450 family, a family of cytochromes that absorb light maximally at 450 nm when complexed in vitro with exogenous carbon monoxide. These membrane-
791
Because the hydroxylation reactions promoted by P450 enzymes are oxidation reactions, it is at first glance surprising that they also consume the reductant NADPH. NADPH transfers its high-
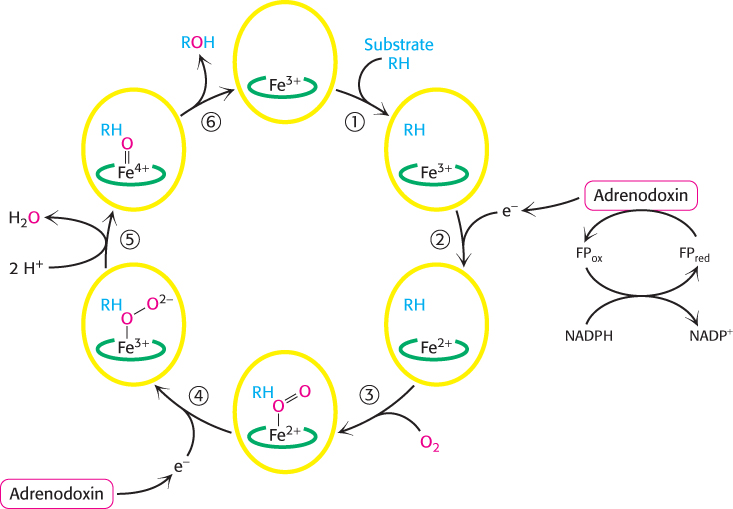
Without the addition of this electron, P450 will not bind oxygen. Recall that only the ferrous form (Fe2+) of myoglobin and hemoglobin binds oxygen (Section 7.1). The binding of O2 to the heme is followed by the acceptance of a second electron from adrenodoxin. The acceptance of this second electron leads to cleavage of the O—
The cytochrome P450 system is widespread and performs a protective function
 The cytochrome P450 system, which in mammals is located primarily in the smooth endoplasmic reticulum of the liver and small intestine, is also important in the detoxification of foreign substances (xenobiotic compounds). For example, the hydroxylation of phenobarbital, a barbiturate, increases its solubility and facilitates its excretion. Likewise, polycyclic aromatic hydrocarbons that are ingested by drinking contaminated water are hydroxylated by P450, providing sites for conjugation with highly polar units (e.g., glucuronate or sulfate) that markedly increase the solubility of the modified aromatic molecule. One of the most relevant functions of the cytochrome P450 system to human beings is its role in metabolizing drugs such as caffeine and ibuprofen (Chapter 36). Some members of the cytochrome P450 system also metabolize ethanol (Section 27.6). The duration of action of many medications depends on their rate of inactivation by the P450 system. Despite its general protective role in the removal of foreign chemicals, the action of the P450 system is not always beneficial. Some of the most powerful carcinogens are generated from harmless compounds by the P450 system in vivo in the process of metabolic activation (Figure 28.34). In plants, the cytochrome P450 system plays a role in the synthesis of toxic compounds as well as the pigments of flowers.
The cytochrome P450 system, which in mammals is located primarily in the smooth endoplasmic reticulum of the liver and small intestine, is also important in the detoxification of foreign substances (xenobiotic compounds). For example, the hydroxylation of phenobarbital, a barbiturate, increases its solubility and facilitates its excretion. Likewise, polycyclic aromatic hydrocarbons that are ingested by drinking contaminated water are hydroxylated by P450, providing sites for conjugation with highly polar units (e.g., glucuronate or sulfate) that markedly increase the solubility of the modified aromatic molecule. One of the most relevant functions of the cytochrome P450 system to human beings is its role in metabolizing drugs such as caffeine and ibuprofen (Chapter 36). Some members of the cytochrome P450 system also metabolize ethanol (Section 27.6). The duration of action of many medications depends on their rate of inactivation by the P450 system. Despite its general protective role in the removal of foreign chemicals, the action of the P450 system is not always beneficial. Some of the most powerful carcinogens are generated from harmless compounds by the P450 system in vivo in the process of metabolic activation (Figure 28.34). In plants, the cytochrome P450 system plays a role in the synthesis of toxic compounds as well as the pigments of flowers.
792
 Earlier (Section 9.1), we examined the use of protease inhibitors to treat HIV infection. These inhibitors, in conjunction with inhibitors of other HIV enzymes, have drastically reduced deaths due to AIDS. The effectiveness of these drugs is sometimes compromised because P450 enzymes, such as cytochrome P450-
Earlier (Section 9.1), we examined the use of protease inhibitors to treat HIV infection. These inhibitors, in conjunction with inhibitors of other HIV enzymes, have drastically reduced deaths due to AIDS. The effectiveness of these drugs is sometimes compromised because P450 enzymes, such as cytochrome P450-
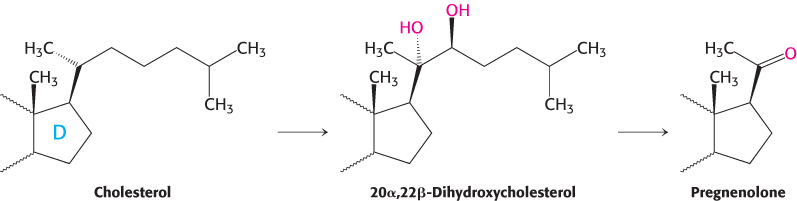
Pregnenolone, a precursor of many other steroids, is formed from cholesterol by cleavage of its side chain
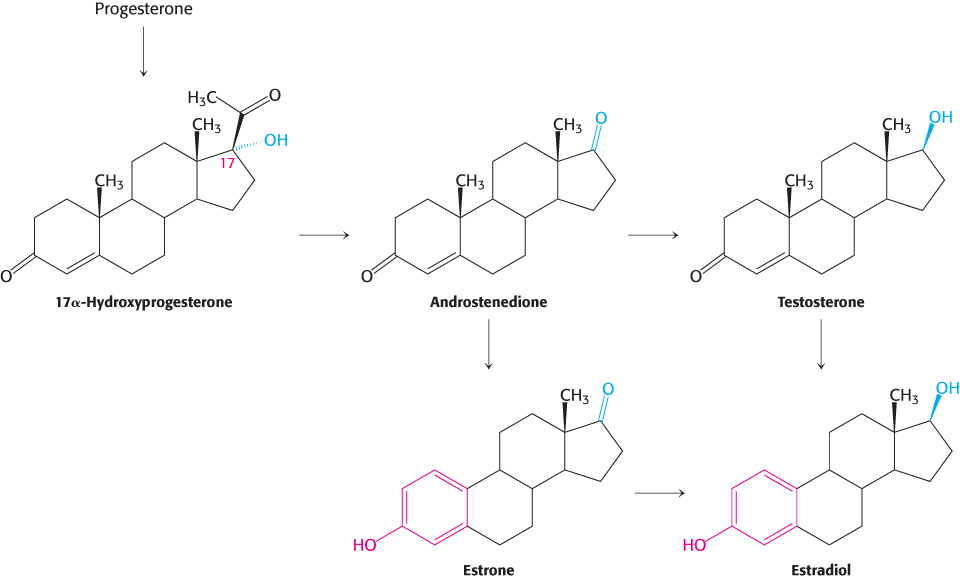
Steroid hormones contain 21 or fewer carbon atoms, whereas cholesterol contains 27. Thus, the first stage in the synthesis of steroid hormones is the removal of a six-
Progesterone and corticosteroids are synthesized from pregnenolone
Progesterone is synthesized from pregnenolone in two steps. The 3-
Androgens and estrogens are synthesized from pregnenolone
Androgens and estrogens also are synthesized from pregnenolone through the intermediate progesterone. Androgens contain 19 carbon atoms. The synthesis of androgens starts with the hydroxylation of progesterone at C-
793
Estrogens are synthesized from androgens by the loss of the C-
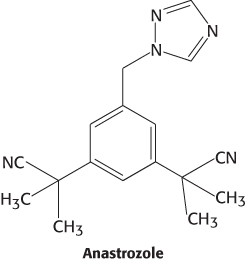
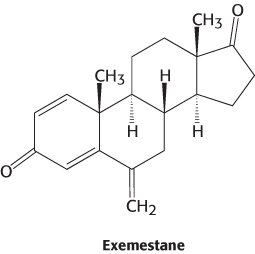
 Because breast and ovarian cancers frequently depend on estrogens for growth, aromatase inhibitors are often used as a treatment for these cancers. Anastrozole is a competitive inhibitor of the enzyme, whereas exemestane is a suicide inhibitor that covalently modifies and inactivates the enzyme.
Because breast and ovarian cancers frequently depend on estrogens for growth, aromatase inhibitors are often used as a treatment for these cancers. Anastrozole is a competitive inhibitor of the enzyme, whereas exemestane is a suicide inhibitor that covalently modifies and inactivates the enzyme.
794
Vitamin D is derived from cholesterol by the ring-splitting activity of light
Cholesterol is also the precursor of vitamin D, which plays an essential role in the control of calcium and phosphorus metabolism. 7-
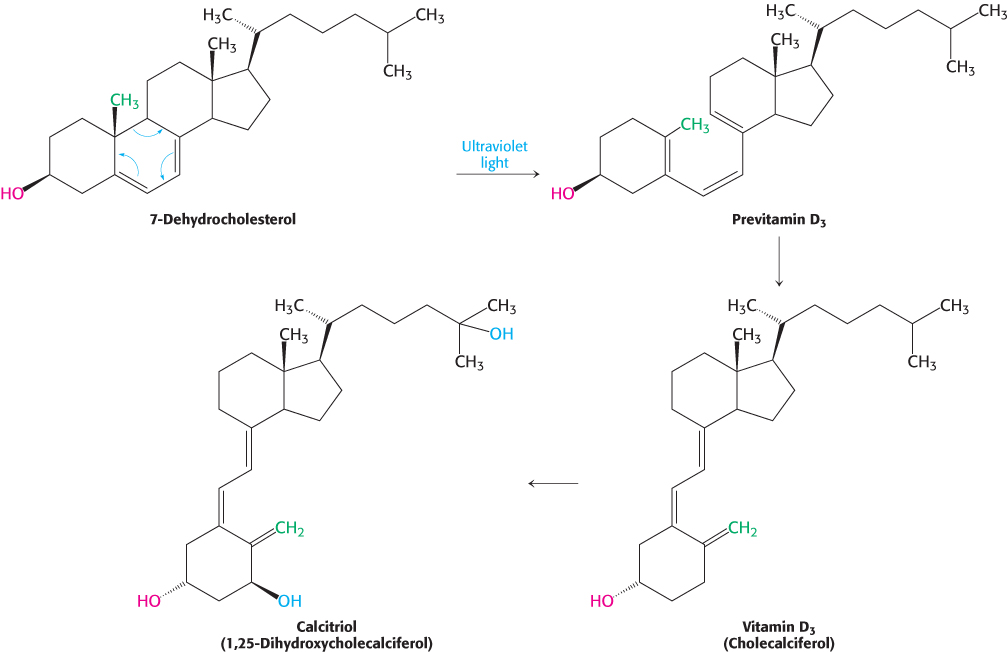
795
 Vitamin D deficiency in childhood produces rickets, a disease characterized by inadequate calcification of cartilage and bone. Rickets was so common in seventeenth-
Vitamin D deficiency in childhood produces rickets, a disease characterized by inadequate calcification of cartilage and bone. Rickets was so common in seventeenth-
Research over the past few years indicates that vitamin D may play a much larger biochemical role than simply the regulation of bone metabolism. Muscle seems to be a target of vitamin D action. In muscle, vitamin D appears to affect a number of biochemical processes with the net effect being enhanced muscle performance. Studies also suggest that vitamin D prevents cardiovascular disease, reduces the incidence of a variety of cancers, and protects against autoimmune diseases including diabetes. Moreover, vitamin D deficiency appears to be more common than previously thought. In the United States, 75% of Blacks and many Hispanics and Asians have insufficient blood levels of vitamin D. This recent research on vitamin D shows again the dynamic nature of biochemical investigations. Vitamin D, a chemical whose biochemical role was believed to be well established, now offers new frontiers of biomedical research.