30.4 Eukaryotic Protein Synthesis Differs from Bacterial Protein Synthesis Primarily in Translation Initiation
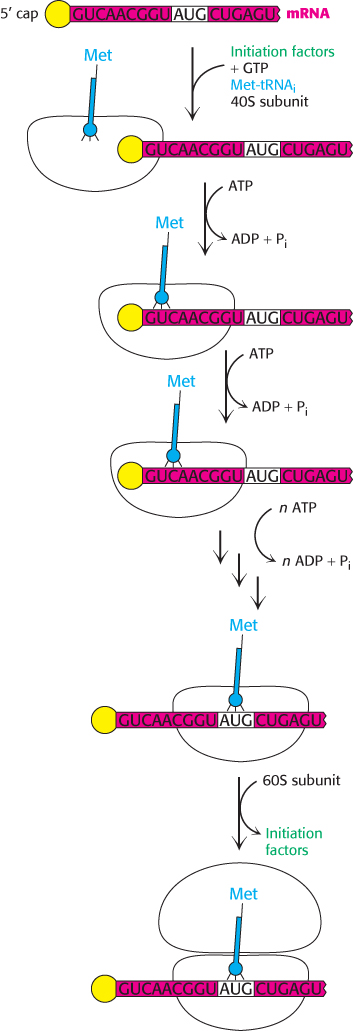
Figure 30.27: Eukaryotic translation initiation. In eukaryotes, translation initiation starts with the assembly of a complex on the 5′ cap that includes the 40S subunit and Met-tRNAi. Driven by ATP hydrolysis, this complex scans the mRNA until the first AUG is reached. The 60S subunit is then added to form the 80S initiation complex.
The basic plan of protein synthesis in eukaryotes and archaea is similar to that in bacteria. The major structural and mechanistic themes recur in all domains of life. However, eukaryotic protein synthesis entails more protein components than does bacterial protein synthesis, and some steps are more intricate. Some noteworthy similarities and differences are as follows:
1. Ribosomes. Eukaryotic ribosomes are larger. They consist of a 60S large subunit and a 40S small subunit, which come together to form an 80S particle having a mass of 4200 kDa, compared with 2700 kDa for the bacterial 70S ribosome. The 40S subunit contains an 18S RNA that is homologous to the bacterial 16S RNA. The 60S subunit contains three RNAs: the 5S RNA, which is homologous to the bacterial 5S rRNA; the 28S RNA, which is homologous to the bacterial 23S molecules; and the 5.8S RNA, which is homologous to the 5′ end of the 23S RNA of bacteria.
2. Initiator tRNA. In eukaryotes, the initiating amino acid is methionine rather than N-formylmethionine. However, as in bacteria, a special tRNA participates in initiation. This aminoacyl-tRNA is called Met-tRNAi (the subscript “i” stands for initiation).
3. Initiation. The initiating codon in eukaryotes is always AUG. Eukaryotes, in contrast with bacteria, do not have a specific purine-rich sequence on the 5′ side to distinguish initiator AUGs from internal ones. Instead, the AUG nearest the 5′ end of mRNA is usually selected as the start site. Eukaryotes utilize many more initiation factors than do bacteria, and their interplay is much more complex. The prefix eIF denotes a eukaryotic initiation factor.
Initiation begins with the formation of a ternary complex consisting of the 40S ribosome and Met-tRNAi in association with eIF-2. The complex is called the 43S preinitiation complex (PIC). The PIC binds to the 5′ end of mRNA and begins searching for an AUG codon by moving step-by-step in the 3′ direction. Initiation factor eIF-4E binds to the 5′-cap of the mRNA (Section 29.3) and facilitates binding of PIC to the mRNA (Figure 30.27). This scanning process is catalyzed by helicases that move along the mRNA powered by ATP hydrolysis. Pairing of the anticodon of Met-tRNAi with the AUG codon of mRNA signals that the target has been found. In almost all cases, eukaryotic mRNA has only one start site and hence is the template for only a single protein. In contrast, a bacterial mRNA can have multiple Shine–Dalgarno sequences and, hence, start sites, and it can serve as a template for the synthesis of several proteins.
The difference in initiation mechanism between bacteria and eukaryotes is, in part, a consequence of the difference in RNA processing. The 5′ end of mRNA is readily available to ribosomes immediately after transcription in bacteria. In contrast, in eukaryotes pre-mRNA must be processed and transported to the cytoplasm before translation is initiated. The 5′ cap provides an easily recognizable starting point. In addition, the complexity of eukaryotic translation initiation provides another mechanism for regulation of gene expression that we shall explore further in Chapter 31.
Although most eukaryotic mRNA molecules rely on the 5′ cap to initiate protein synthesis, recent work has established that some mRNA molecules can recruit ribosomes for initiation without the use of a 5′-cap and cap-binding proteins. In these mRNAs, highly structured RNA sequences called internal ribosome entry sites (IRES) facilitate 40S ribosome binding to the mRNA. IRES were first discovered in the genomes of RNA viruses and have since been found in other viruses, as well as in a subset of cellular mRNA that appears to take part in development and cell stress. The molecular mechanism by which IRES function to initiate protein synthesis remains to be determined.
4. The Structure of mRNA. Soon after the PIC binds the mRNA, eIF-4G links eIF-4E to a protein associated with the poly(A) tail, the poly(A)-binding protein I (PABPI; Figure 30.28). Cap and tail are thus brought together to form a circle of mRNA. The benefits of circularization of mRNA remain to be determined, but a requirement for circularization may prevent translation of mRNA molecules that have lost their poly(A) tails (Section 29.3).
5. Elongation and Termination. Eukaryotic elongation factors EF1α and EF1βγ are the counterparts of bacterial EF-Tu and EF-Ts. The GTP form of EF1α delivers aminoacyl-tRNA to the A site of the ribosome, and EF1βγ catalyzes the exchange of GTP for bound GDP. Eukaryotic EF2 mediates GTP-driven translocation in much the same way as does bacterial EF-G. Termination in eukaryotes is carried out by a single release factor, eRF1, compared with two in bacteria. Release factor eIF-3 accelerates the activity of eRF-1.
6. Organization. The components of the translation machinery in higher eukaryotes are organized into large complexes associated with the cytoskeleton. This association is believed to facilitate the efficiency of protein synthesis. Recall that organization of elaborate biochemical processes into physical complexes is a reoccurring theme in biochemistry (Section 18.5 and Section 25.2).
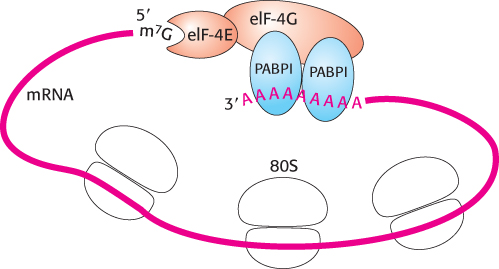
Figure 30.28: Protein interactions circularize eukaryotic mRNA.
[Information from H. Lodish et al., Molecular Cell Biology, 5th ed. (W. H. Freeman and Company, 2004), Fig. 4.31.]
Mutations in initiation factor 2 cause a curious pathological condition
 Mutations in eukaryotic initiation factor 2 result in a mysterious disease, called vanishing white matter (VWM) disease, in which nerve cells in the brain disappear and are replaced by cerebrospinal fluid (Figure 30.29). The white matter of the brain consists predominantly of nerve axons that connect the gray matter of the brain to the rest of the body. Death, resulting from fever or extended coma, can occur anywhere from a few years to decades after the onset of the disease. Symptoms usually appear in young children but can develop shortly after birth or even in adulthood. An especially puzzling aspect of the disease is its tissue specificity. A mutation in a biochemical process as fundamental to life as protein-synthesis initiation would be predicted to be lethal or at least to affect all tissues of the body. Diseases such as VWM graphically show that, although much progress has been made in biochemistry, much more research will be required to understand the complexities of health and disease.
Mutations in eukaryotic initiation factor 2 result in a mysterious disease, called vanishing white matter (VWM) disease, in which nerve cells in the brain disappear and are replaced by cerebrospinal fluid (Figure 30.29). The white matter of the brain consists predominantly of nerve axons that connect the gray matter of the brain to the rest of the body. Death, resulting from fever or extended coma, can occur anywhere from a few years to decades after the onset of the disease. Symptoms usually appear in young children but can develop shortly after birth or even in adulthood. An especially puzzling aspect of the disease is its tissue specificity. A mutation in a biochemical process as fundamental to life as protein-synthesis initiation would be predicted to be lethal or at least to affect all tissues of the body. Diseases such as VWM graphically show that, although much progress has been made in biochemistry, much more research will be required to understand the complexities of health and disease.
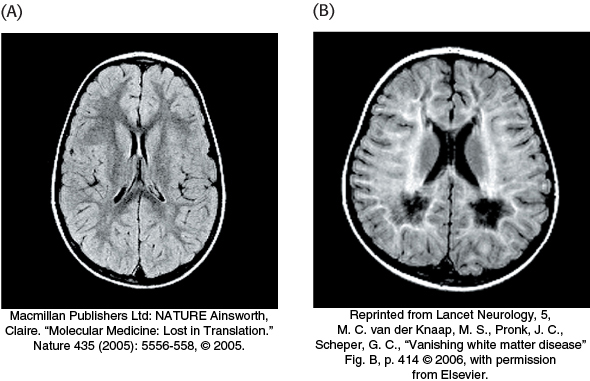
Figure 30.29: The effects of vanishing white matter disease. (A) In the normal brain, magnetic resonance imaging (MRI) visualizes the white matter as dark gray. (B) In the diseased brain, this MRI reveals that white matter is replaced by cerebrospinal fluid, seen as white.


 Mutations in eukaryotic initiation factor 2 result in a mysterious disease, called vanishing white matter (VWM) disease, in which nerve cells in the brain disappear and are replaced by cerebrospinal fluid (Figure 30.29). The white matter of the brain consists predominantly of nerve axons that connect the gray matter of the brain to the rest of the body. Death, resulting from fever or extended coma, can occur anywhere from a few years to decades after the onset of the disease. Symptoms usually appear in young children but can develop shortly after birth or even in adulthood. An especially puzzling aspect of the disease is its tissue specificity. A mutation in a biochemical process as fundamental to life as protein-
Mutations in eukaryotic initiation factor 2 result in a mysterious disease, called vanishing white matter (VWM) disease, in which nerve cells in the brain disappear and are replaced by cerebrospinal fluid (Figure 30.29). The white matter of the brain consists predominantly of nerve axons that connect the gray matter of the brain to the rest of the body. Death, resulting from fever or extended coma, can occur anywhere from a few years to decades after the onset of the disease. Symptoms usually appear in young children but can develop shortly after birth or even in adulthood. An especially puzzling aspect of the disease is its tissue specificity. A mutation in a biochemical process as fundamental to life as protein-