31.3 Regulatory Circuits Can Result in Switching Between Patterns of Gene Expression
The study of viruses that infect bacteria has led to significant advances in our understanding of the processes that control gene expression. Again, sequence-specific DNA-binding proteins play key roles in these processes. Investigations of bacteriophage λ have been particularly revealing. We examined the alternative infection modes of λ phage in Chapter 5. In the lytic pathway, most of the genes in the viral genome are transcribed, initiating the production of many virus particles and leading to the eventual lysis of the bacterial cell with the concomitant release of approximately 100 virus particles. In the lysogenic pathway, the viral genome is incorporated into the bacterial DNA where most of the viral genes remain unexpressed, allowing the viral genome to be carried along as the bacteria replicate. Two key proteins and a set of regulatory sequences in the viral genome are responsible for the switch that determines which of these two pathways is followed.
The λ repressor regulates its own expression
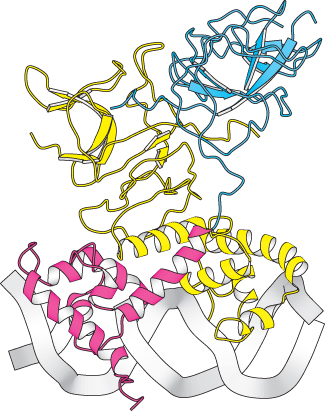
Figure 31.14:  Structure of the λ repressor bound to DNA. The λ repressor binds to DNA as a dimer. The amino-terminal domain of one subunit is shown in red and the carboxyl-terminal domain is shown in blue. In the other subunit, both domains are shown in yellow. Notice how a helices on the amino-terminal domains fit into the major groove of the DNA.
Structure of the λ repressor bound to DNA. The λ repressor binds to DNA as a dimer. The amino-terminal domain of one subunit is shown in red and the carboxyl-terminal domain is shown in blue. In the other subunit, both domains are shown in yellow. Notice how a helices on the amino-terminal domains fit into the major groove of the DNA.
[Drawn from 3DBN.pdb.]
The first protein that we shall consider is the λ repressor, sometimes known as the λ cI protein. This protein is key because it blocks, either directly or indirectly, the transcription of almost all genes encoded by the virus. The one exception is the gene that encodes the λ repressor itself. The λ repressor consists of an amino-terminal DNA-binding domain and a carboxyl-terminal domain that participates in protein oligomerization (Figure 31.14). This protein binds to a number of key sites in the λ phage genome. The sites of greatest interest for our present discussion are in the so-called right operator (Figure 31.15). This region includes three binding sites for the λ repressor dimer, as well as two promoters within a region of approximately 80 base pairs. One promoter drives the expression of the gene for the λ repressor itself, whereas the other drives the expression of a number of other viral genes.
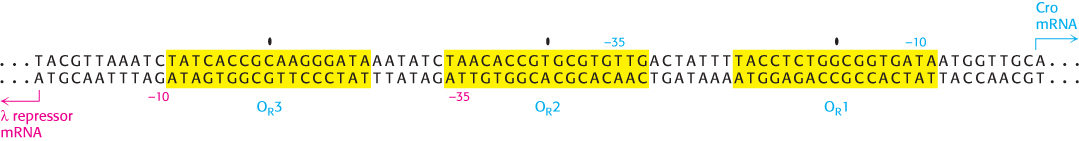
Figure 31.15: Sequence of the λ right operator. The three operator sites (OR1, OR2, and OR3) are shaded yellow with their centers indicated. The start sites for the λ repressor mRNA and the Cro mRNA are indicated, as are their −10 and −35 sequences.
The λ repressor does not have the same affinity for the three sites; it binds the site OR1 with the highest affinity. In addition, the binding to adjacent sites is cooperative so that, after a λ repressor dimer has bound at OR1, the likelihood that a protein will bind to the adjacent site OR2 increases by approximately 25-fold. Thus, when λ repressor is present in the cell at moderate concentrations, the most likely configuration has λ repressor bound at OR1 and OR2, but not at OR3. In this configuration, the λ repressor dimer bound at OR1 blocks access to the promoter on the right side of the operator sites, repressing transcription of the adjacent gene, which encodes a protein termed Cro (controller of repressor and others), while the repressor dimer at OR2 can be in contact with RNA polymerase and stimulate transcription of the promoter that controls the transcription of the gene that encodes the λ repressor itself.
Thus, the λ repressor stimulates its own production. As the concentration of the λ repressor increases further, an additional repressor dimer can bind to the OR3 site, blocking the other promoter and repressing the production of additional repressor. Thus, the right operator serves to maintain the λ repressor in a narrow, stable concentration range (Figure 31.16). The λ repressor also blocks other promoters in the λ phage genome so that the repressor is the only phage protein produced, which corresponds to the lysogenic state.
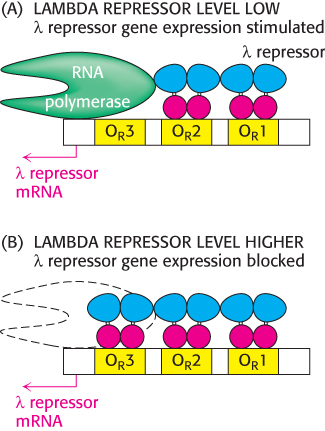
Figure 31.16: The λ repressor controls its own synthesis. (A) When λ repressor levels are relatively low, the repressor binds to sites OR1 and OR2 and stimulates the transcription of the gene that encodes the λ repressor itself. (B) When λ repressor levels are higher, the repressor also binds to site OR3, blocking access to its promoter and repressing transcription from this gene.
A circuit based on the λ repressor and Cro forms a genetic switch
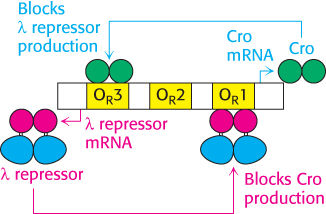
Figure 31.17: The λ repressor and Cro form a genetic circuit. The λ repressor blocks the production of Cro by binding most favorably to site OR1 whereas Cro blocks the production of the λ repressor by binding most favorably to site OR3. This circuit forms a switch that determines whether the lysogenic or the lytic pathway is followed.
What stimulates the switch to the lytic pathway? Changes such as DNA damage initiate the cleavage of the λ repressor at a specific bond between the DNA-binding and oligomerization domains. This process is mediated by the E. coli RecA protein (Section 28.5). After this cleavage has taken place, the affinity of the λ repressor for DNA is reduced. After the λ repressor is no longer bound to the OR1 site, the Cro gene can be transcribed. Cro is a small protein that binds to the same sites as the λ repressor does, but with a different order of affinity for the three sites in the right operator. In particular, Cro has the highest affinity for OR3. Cro bound in this site blocks the production of new repressor. The absence of repressor leads to the production of other phage genes, leading to the production of virus particles and the eventual lysis of the host cells. Thus, this genetic circuit acts as a switch with two stable states: (1) repressor high, Cro low, corresponding to the lysogenic state, and (2) Cro high, repressor low, corresponding to the lytic state (Figure 31.17). Regulatory circuits with different DNA-binding proteins controlling the expression of each other’s genes constitute a common motif for controlling gene expression.
Many prokaryotic cells release chemical signals that regulate gene expression in other cells
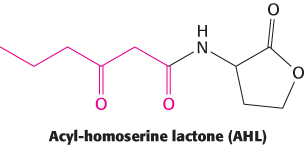
Figure 31.18: Autoinducer structure. The structure of the acyl-homoserine lactone N-3-oxo-hexanoyl homoserine lactone, the autoinducer from V. fischeri. The autoinducers from other bacterial species can have different acyl groups (shown in red).
Prokaryotic cells have been traditionally viewed as solitary single cells. However, it is becoming increasingly clear that, in many circumstances, prokaryotic cells live in complex communities, interacting with other cells of their own and different species. These social interactions change the patterns of gene expression within the cells.
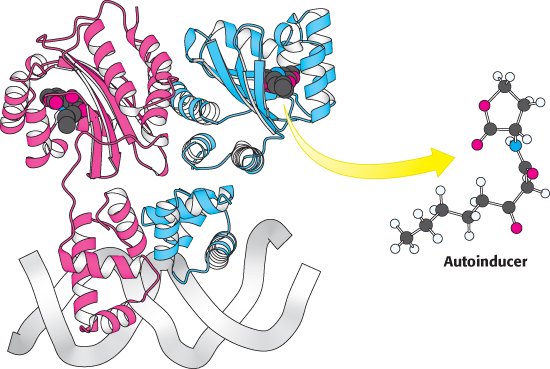
Figure 31.19: Quorum-sensing gene regulator. The structure of a homolog of LuxR (TraR from the bacterium Agrobacterium tumefaciens) is shown. Notice that the dimeric protein binds to DNA through an a-helical domain, whereas the autoinducer binds to a separate domain.
An important type of interaction is called quorum sensing. This phenomenon was discovered in the bacteria Vibrio fischeri, a species of bacterium that can live inside a specialized light organ in the bobtail squid. In this symbiotic relation, the bacteria produce luciferase and bioluminesce, providing protection for the squid (by preventing being backlit by moonlight) in exchange for a safer place to live and reproduce. When these bacteria are grown in culture at low density, they are not bioluminescent. However, when the cell density reaches a critical level, the gene for luciferase is expressed and the cells bioluminesce. A key observation was that, when V. fischeri cells were transferred to a sterile medium in which other V. fischeri cells had been grown to high density, the cells became bioluminescent even at low cell density. This experiment revealed that a chemical, subsequently shown to be N-3-oxo-hexanoyl homoserine lactone (hereafter AHL for acyl-homoserine lactone), had been released into the medium where it triggered the development of the bioluminescence (Figure 31.18). This compound and other compounds that play similar roles are termed autoinducers.
Cells of V. fischeri release the autoinducer into their environment and other V. fischeri cells take up the chemical. V. fischeri cells express a DNA-binding protein LuxR that serves as the receptor for the autoinducer. LuxR comprises two domains, one of which binds AHL and the other of which binds DNA through a helix-turn-helix motif (Figure 31.19). After the AHL concentration inside the cell has increased to an appropriate level, a substantial fraction of the LuxR molecules bind AHL. When bound to AHL, LuxR dimers bind to specific sites on DNA and increase the rate of transcription initiation at specific genes. The target genes include an operon that contains LuxA and LuxB, which together encode the luciferase enzyme, and LuxI, which produces an enzyme that catalyzes the formation of more AHL.
Because each cell produces only a small amount of the autoinducer, this regulatory system allows each V. fischeri cell to determine the density of the V. fischeri population in its environment—hence the term quorum sensing for this process. Studies of other prokaryotic cells are revealing an elaborate chemical language of different autoinducers (as well as autorepressors that repress specific genes). The “words” in this language include other acyl-homoserine lactone molecules with different acyl chain lengths and functionalities, as well as other distinct classes of molecules.

 Structure of the λ repressor bound to DNA. The λ repressor binds to DNA as a dimer. The amino-
Structure of the λ repressor bound to DNA. The λ repressor binds to DNA as a dimer. The amino-



