34.1 Antibodies Possess Distinct Antigen-Binding and Effector Units
Antibodies are central molecular players in the immune response. In Chapter 3, we discussed the application of antibodies as tools to study proteins; let us now consider the native structures and functions of these remarkable molecules. In 1959, Rodney Porter showed that immunoglobulin G (IgG), the major antibody in serum, can be cleaved into three 50-kDa fragments by the limited proteolytic action of papain. Two of these fragments bind antigen. They are called Fab (F stands for fragment, ab for antigen binding). The other fragment, called Fc because it crystallizes readily, does not bind antigen, but it has other important biological activities, including the mediation of responses termed effector functions. These functions include the initiation of the complement cascade, a process that leads to the lysis of target cells. Although such effector functions are crucial to the functioning of the immune system, they will not be considered further here.
How do these fragments relate to the three-dimensional structure of whole IgG molecules? Immunoglobulin G consists of two kinds of polypeptide chains, a 25-kDa light (L) chain and a 50-kDa heavy (H) chain (Figure 34.5). The subunit composition is L2H2. Each L chain is linked to an H chain by a disulfide bond, and the H chains are linked to each other by at least one disulfide bond. Examination of the amino acid sequences and three-dimensional structures of IgG molecules reveals that each L chain comprises two homologous domains, termed immunoglobulin domains, to be described in detail in Section 34.2. Each H chain has four immunoglobulin domains. Overall, the molecule adopts a conformation that resembles the letter Y. The stem of this Y, corresponding to the Fc fragment obtained by cleavage with papain, consists of the two carboxyl-terminal immunoglobulin domains of each H chain. The two arms of the Y, corresponding to the two Fab fragments, are formed by the two amino-terminal domains of each H chain and the two domains of each L chain. The linkers between the stem and the two arms consist of extended polypeptide regions within the H chains and are quite flexible.
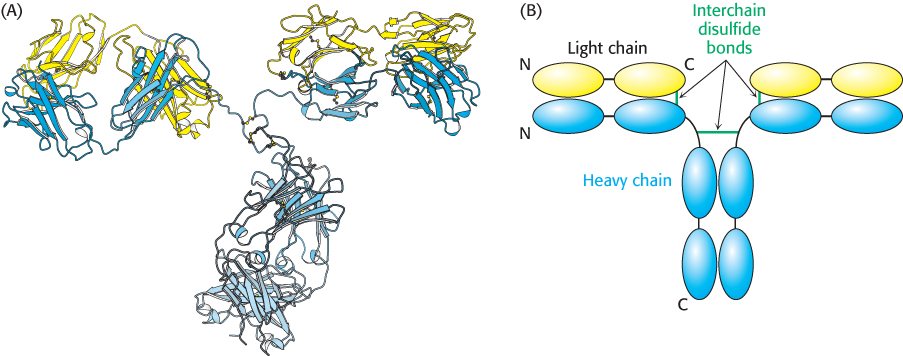
Figure 34.5:  Immunoglobulin G structure. (A) The three-dimensional structure of an IgG molecule showing the light chains in yellow and the heavy chains in blue. (B) A schematic view of an IgG molecule indicating the positions of the interchain disulfide bonds. Abbreviations: N, amino terminus; C, carboxyl terminus.
Immunoglobulin G structure. (A) The three-dimensional structure of an IgG molecule showing the light chains in yellow and the heavy chains in blue. (B) A schematic view of an IgG molecule indicating the positions of the interchain disulfide bonds. Abbreviations: N, amino terminus; C, carboxyl terminus.
[Drawn from 1IGT.pdb.]
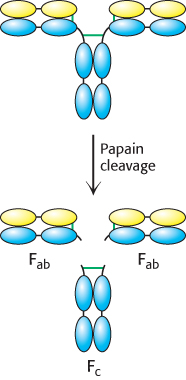
Figure 34.6: Immunoglobulin G cleavage. Treatment of intact IgG molecules with the protease papain results in the formation of three large fragments: two Fab fragments that retain antigen-binding capability and one Fc fragment that does not.
Papain cleaves the H chains on the carboxyl-terminal side of the disulfide bond that links each L and H chain (Figure 34.6). Thus, each Fab consists of an entire L chain and the amino-terminal half of an H chain, whereas Fc consists of the carboxyl-terminal halves of both H chains. Each Fab contains a single antigen-binding site. Because an intact IgG molecule contains two Fab components and therefore has two binding sites, it can cross-link multiple antigens (Figure 34.7). Furthermore, the Fc and the two Fab units of the intact IgG are joined by flexible polypeptide regions that allow facile variation in the angle between the Fab units through a wide range (Figure 34.8). This kind of mobility, called segmental flexibility, can enhance the formation of an antibody–antigen complex by enabling both recognition sites on an antibody to bind an antigen that possesses multiple binding sites, such as a viral coat composed of repeating identical monomers. The sites at the tips of the Fab units simply move to match the distance between specific determinants on the antigen.
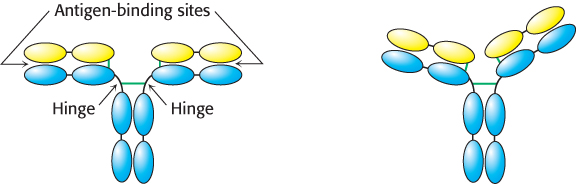
Figure 34.8: Segmental flexibility. The linkages between the Fab and the Fc regions of an IgG molecule are flexible, allowing the two antigen-binding sites to adopt a range of orientations with respect to each other. This flexibility allows effective interactions with a multivalent antigen without requiring that the epitopes on the target be a precise distance apart.
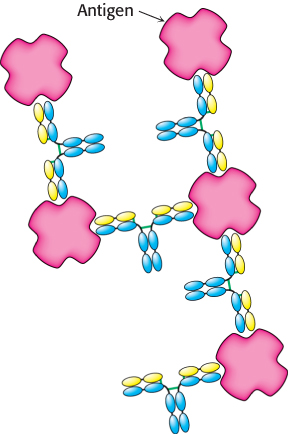
Figure 34.7: Antigen cross-linking. Because IgG molecules include two antigen-binding sites, antibodies can cross-link multivalent antigens such as viral surfaces.
Immunoglobulin G is the antibody present in highest concentration in the serum, but other classes of immunoglobulin also are present (Table 34.2). Each class includes an L chain (either κ or λ) and a distinct H chain (Figure 34.9). The heavy chains in IgG are called γ chains, whereas those in immunoglobulins A, M, D, and E are called α, μ, δ, and ε, respectively. Immunoglobulin M (IgM) is the first class of antibody to appear in the serum after exposure to an antigen. The presence of 10 antigen recognition sites enables IgM to bind especially tightly to antigens containing multiple identical epitopes. The strength of an interaction comprising multiple independent binding interactions between partners is termed avidity rather than affinity, which denotes the binding strength of a single binding site.
|
|
Serum concentration (mg ml−1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note: n = 1, 2, or 3. IgM and oligomers of IgA also contain J chains that connect immunoglobulin molecules. IgA in secretions has an additional component. |
Table 34.2: Properties of immunoglobulin classes
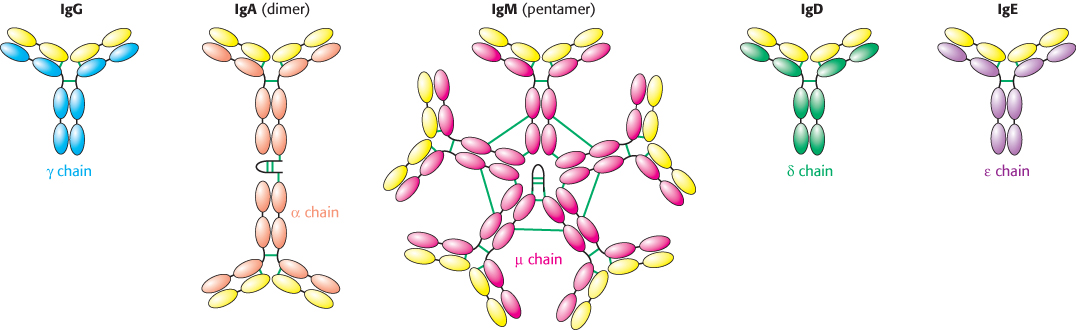
Figure 34.9: Classes of immunoglobulin. Each of five classes of immunoglobulin has the same light chain (shown in yellow) combined with a different heavy chain (γ, α, μ, δ, or ´). Disulfide bonds are indicated by green lines. The IgA dimer and the IgM pentamer have a small polypeptide chain in addition to the light and heavy chains.
Immunoglobulin A (IgA) is the major class of antibody in external secretions, such as saliva, tears, bronchial mucus, and intestinal mucus. Thus, IgA serves as a first line of defense against bacterial and viral antigens. The role of immunoglobulin D (IgD) has long been a mystery, but recent studies suggest IgD plays a role in the activation of basophils, white blood cells that have antiparasitic functions. Immunoglobulin E (IgE) is important in conferring protection against parasites, but IgE also participates in allergic reactions. IgE–antigen complexes form cross-links with receptors on the surfaces of mast cells to trigger a cascade that leads to the release of granules containing pharmacologically active molecules. Histamine, one of the agents released, induces smooth-muscle contraction and stimulates the secretion of mucus.

 Immunoglobulin G structure. (A) The three-
Immunoglobulin G structure. (A) The three-


