34.4 Major-Histocompatibility-Complex Proteins Present Peptide Antigens on Cell Surfaces for Recognition by T-Cell Receptors
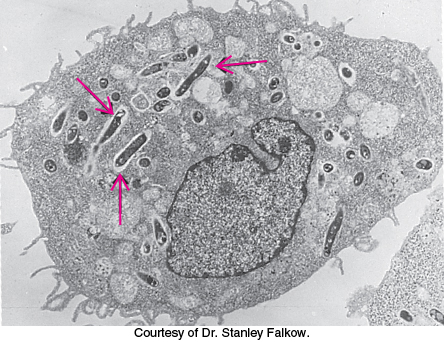
Figure 34.24: Intracellular pathogen. An electron micrograph showing mycobacteria (arrows) inside an infected macrophage.
Soluble antibodies are highly effective against extracellular pathogens, but they confer little protection against microorganisms that are predominantly intracellular, such as some viruses and mycobacteria (which cause tuberculosis and leprosy). These pathogens are shielded from antibodies by the host-cell membrane (Figure 34.24). A different and more subtle strategy, cell-mediated immunity, evolved to cope with intracellular pathogens. T cells continually scan the surfaces of all cells and kill those that exhibit foreign markings. The task is not simple; intracellular microorganisms are not so obliging as to intentionally leave telltale traces on the surface of their host. Quite the contrary, successful pathogens are masters of the art of camouflage. Vertebrates have evolved an ingenious mechanism—cut and display—to reveal the presence of stealthy intruders. Nearly all vertebrate cells exhibit on their surfaces a sample of peptides derived from the digestion of proteins in their cytoplasm. These peptides are displayed by integral membrane proteins that are encoded by the major histocompatibility complex (MHC). Specifically, peptides derived from cytoplasmic proteins are bound to and displayed by class I MHC proteins. The dendritic cells of the innate immune system that subject pathogens to phagocytosis migrate to lymphatic tissue, where they use an MHC-like mechanism to present foreign peptides or lipid components to T cells—thus linking the innate and adaptive immune responses to pathogens.
How are these peptides generated and delivered to the plasma membrane? The process starts in the cytoplasm with the degradation of proteins—self-proteins as well as those of pathogens (Figure 34.25). Digestion is carried out by proteasomes (Section 23.2). The resulting peptide fragments are transported from the cytoplasm into the lumen of the endoplasmic reticulum by the TAP protein (for transporter associated with antigen processing), a member of the ABC transporter family of ATP-driven pumps (Section 13.2). In the ER, peptides combine with nascent class I MHC proteins; these complexes are then targeted to the plasma membrane.
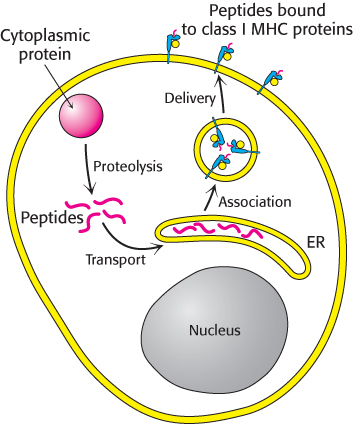
Figure 34.25: Presentation of peptides from cytoplasmic proteins. Class I MHC proteins on the surfaces of most cells display peptides that are derived from cytoplasmic proteins by proteolysis.
MHC proteins embedded in the plasma membrane tenaciously grip their bound peptides so that they can be touched and scrutinized by T-cell receptors on the surface of a killer cell. Foreign peptides bound to class I MHC proteins signal that a cell is infected and mark it for destruction by cytotoxic T cells. An assembly consisting of the foreign peptide—MHC complex, the T-cell receptor, and numerous accessory proteins—triggers a cascade that induces apoptosis in the infected cell. Strictly speaking, infected cells are not killed but, instead, are triggered to commit suicide to benefit the host.
Peptides presented by MHC proteins occupy a deep groove flanked by alpha helices
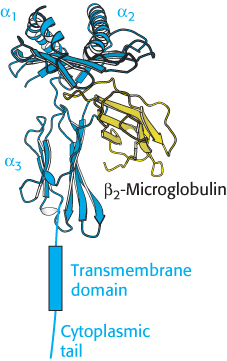
Figure 34.26:  Class I MHC protein. A protein of this class consists of two chains. Notice that the α chain begins with two domains (α1, α2) that include α helices and continues with an immunoglobulin domain (α3), a transmembrane domain, and a cytoplasmic tail. The second chain, β2-microglobulin, adopts an immunoglobulin fold.
Class I MHC protein. A protein of this class consists of two chains. Notice that the α chain begins with two domains (α1, α2) that include α helices and continues with an immunoglobulin domain (α3), a transmembrane domain, and a cytoplasmic tail. The second chain, β2-microglobulin, adopts an immunoglobulin fold.
[Drawn from 1HHK.pdb.]
The three-dimensional structure of a large extracellular fragment of a human MHC class I protein, human leukocyte antigen A2 (HLA-A2), was solved in 1987 by Don Wiley and Pamela Bjorkman. Class I MHC proteins consist of a 44-kDa α chain noncovalently bound to a 12-kDa polypeptide called β2-microglobulin. The α chain has three extracellular domains (α1, α2, and α3), a transmembrane segment, and a tail that extends into the cytoplasm (Figure 34.26). The β2-microglobulin and the α3 domains have immunoglobulin folds, although the pairing of the two domains differs from that in antibodies. The α1 and α2 domains exhibit a novel and remarkable architecture. They associate intimately to form a deep groove that serves as the peptide-binding site (Figure 34.27). The floor of the groove, which is about 25 Å long and 10 Å wide, is formed by eight β strands, four from each domain. A long helix contributed by the α1 domain forms one side, and a helix contributed by the α2 domain forms the other side. This groove is the binding site for the presentation of peptides.
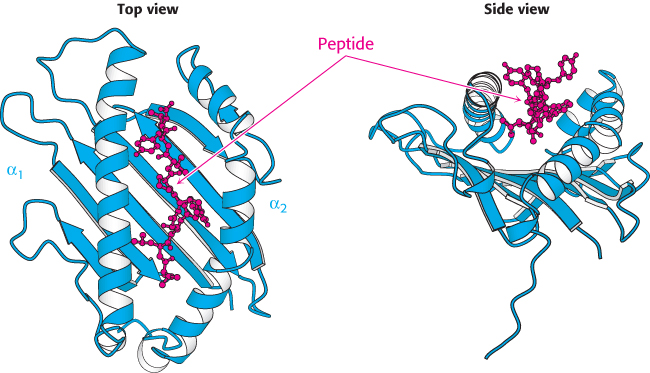
Figure 34.27:  Class I MHC peptide-binding site. The α1 and α2 domains come together to form a groove in which peptides are displayed. Notice that that the peptide is surrounded on three sides by a β sheet and two α helices, but it is accessible from the top of the structure.
Class I MHC peptide-binding site. The α1 and α2 domains come together to form a groove in which peptides are displayed. Notice that that the peptide is surrounded on three sides by a β sheet and two α helices, but it is accessible from the top of the structure.
[Drawn from 1HHK.pdb.]
The groove can be filled by a peptide from 8 to 10 residues long in an extended conformation. MHC proteins are remarkably diverse in the human population; each person expresses as many as six distinct class I MHC proteins, and many different forms are present in different people. The first structure determined, HLA-A2, binds peptides that almost always have leucine in the second position and valine in the last position (Figure 34.28). Side chains from the MHC molecule interact with the amino and carboxyl termini and with the side chains in these two key positions. These two residues are often referred to as the anchor residues. The other residues are highly variable. Thus, many millions of different peptides can be presented by this particular class I MHC protein; the identities of only two of the nine residues are crucial for binding. Each class of MHC molecules requires a unique set of anchor residues. Thus, a tremendous range of peptides can be presented by these molecules. Note that one face of the bound peptide is exposed to solution, where it can be examined by other molecules, particularly T-cell receptors. An additional remarkable feature of MHC–peptide complexes is their kinetic stability; once bound, a peptide is not released, even after a period of days.
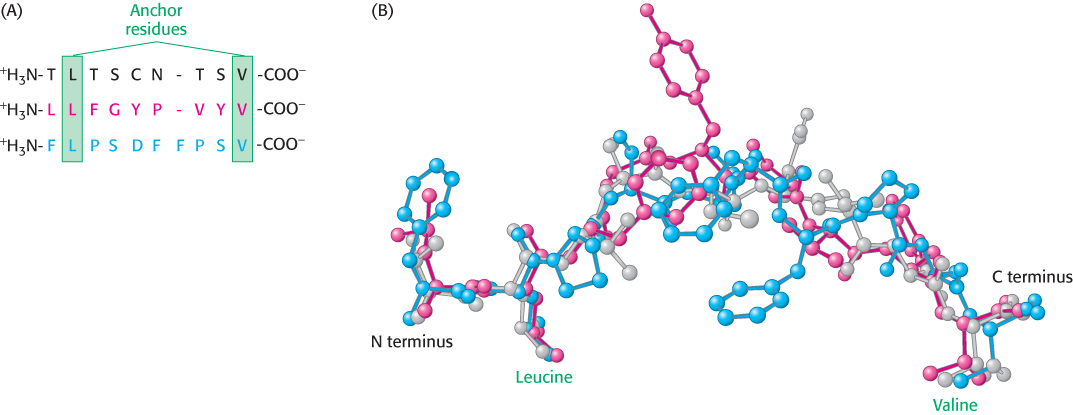
Figure 34.28: Anchor residues. (A) The amino acid sequences of three peptides that bind to the class I MHC protein HLA-A2 are shown. Each of these peptides has leucine in the second position and valine in the carboxyl-terminal position. (B) Comparison of the structures of these peptides reveals that the amino and carboxyl termini, as well as the side chains of the leucine and valine residues, are in essentially the same positions in each peptide, whereas the remainder of the structures are quite different.
T-cell receptors are antibody-like proteins containing variable and constant regions
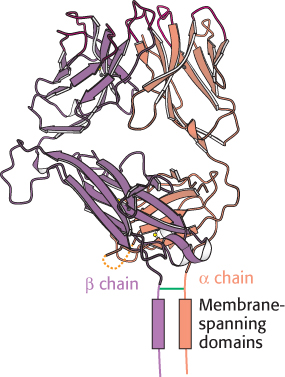
Figure 34.29:  T-cell receptor. This protein consists of an α chain and a β chain, linked by a disulfide bond. Notice that each chain consists of two immunoglobulin domains on the cell surface, a membrane-spanning domain, and a short cytoplasmic tail.
T-cell receptor. This protein consists of an α chain and a β chain, linked by a disulfide bond. Notice that each chain consists of two immunoglobulin domains on the cell surface, a membrane-spanning domain, and a short cytoplasmic tail.
[Drawn from 1BD2.pdb.]
We are now ready to consider the receptor that recognizes peptides displayed by MHC proteins on target cells. The T-cell receptor consists of a 43-kDa α chain joined by a disulfide bond to a 43-kDa β chain (Figure 34.29). Each chain spans the plasma membrane and has a short carboxyl-terminal region on the cytoplasmic side. A small proportion of T cells express a receptor consisting of γ and δ chains in place of α and β. The α and β chains of the T-cell receptor, like immunoglobulin L and H chains, consist of variable and constant regions. Indeed, these domains of the T-cell receptor are homologous to the V and C domains of immunoglobulins. Furthermore, hypervariable sequences present in the V regions of the α and β chains of the T-cell receptor form the binding site for the epitope.
The genetic architecture of these proteins is similar to that of immunoglobulins, though the antibody genetic diversity is distributed over all the CDR loops, whereas T-cell-receptor genetic diversity is concentrated in the CDR3 loop that interacts with the peptide bound to the MHC. The variable region of the T-cell receptor α chain is encoded by about 50 V gene segments and 70 J gene segments. The T-cell receptor β chain is encoded by two D gene segments in addition to 57 V gene segments and 13 J gene segments. Again, the diversity of gene segments and the use of slightly imprecise modes of joining them increase the number of distinct proteins formed. At least 1012 different specificities could arise from combinations of this repertoire of genes. Thus, T-cell receptors, like immunoglobulins, can recognize a very large number of different epitopes. All the receptors on a particular T cell have the same specificity.
How do T cells recognize their targets? The variable regions of the α and β chains of the T-cell receptor form a binding site that recognizes a combined epitope–foreign peptide bound to an MHC protein (Figure 34.30). Neither the foreign peptide alone nor the MHC protein alone forms a complex with the T-cell receptor. Thus, fragments of an intracellular pathogen are presented in a context that allows their detection, leading to the initiation of an appropriate response.
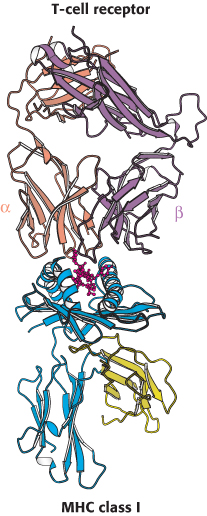
Figure 34.30:  T-cell receptor–class I MHC complex. The T-cell receptor binds to a class I MHC protein containing a bound peptide (red). Notice that the T-cell receptor contacts both the MHC protein and the peptide.
T-cell receptor–class I MHC complex. The T-cell receptor binds to a class I MHC protein containing a bound peptide (red). Notice that the T-cell receptor contacts both the MHC protein and the peptide.
[Drawn from 1BD2.pdb.]
CD8 on cytotoxic T cells acts in concert with T-cell receptors
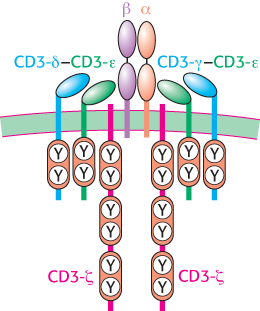
Figure 34.32: T-cell receptor complex. The T-cell receptor is associated with six CD3 molecules: a CD3-γ–CD3-ε heterodimer, a CD3-δ–CD3-γ heterodimer, and two chains of CD3-ζ. Single ITAM sequences are present in the cytoplasmic domains of CD3-γ, CD3-δ, and CD3-ε, whereas three such sequences are found in each CD3-ζ chain.
The T-cell receptor does not act alone in recognizing and mediating the fate of target cells. Cytotoxic T cells also express a protein termed CD8 on their surfaces that is crucial for the recognition of the class I MHC–peptide complex. The abbreviation CD stands for cluster of differentiation, referring to a cell-surface marker that is used to identify a lineage or stage of differentiation. Antibodies specific for particular CD proteins have been invaluable in following the development of leukocytes and in discovering new interactions between specific cell types.
Each chain in the CD8 dimer contains a domain that resembles an immunoglobulin variable domain (Figure 34.31). CD8 interacts primarily with the constant α3 domain of class I MHC proteins. This interaction further stabilizes the interactions between the T cell and its target. The cytoplasmic tail of CD8 contains a docking site for Lck, a cytoplasmic tyrosine kinase akin to Src. The T-cell receptor itself is associated with six polypeptides that form the CD3 complex (Figure 34.32). The γ, δ, and ε chains of CD3 are homologous to Ig-α and Ig-β associated with the B-cell receptor (Figure 34.21); each chain consists of an extracellular immunoglobulin domain and an intracellular ITAM region. These chains associate into CD3-γε and CD3-δε heterodimers. An additional component, the CD3-ζ chain, has only a small extracellular domain and a larger intracellular domain containing three ITAM sequences.
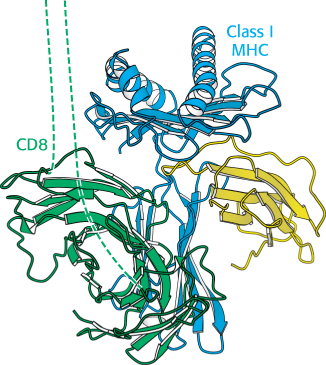
Figure 34.31:  The coreceptor CD8. This dimeric protein extends from the surface of a cytotoxic T cell and binds to class I MHC molecules that are expressed on the surface of the cell that is bound to the T cell. The dashed lines represent extended polypeptide chains that link the immunoglobulin domains of CD8 to the membrane. Notice that the coreceptor interacts primarily with the constant α3 domain of the class I MHC domain.
The coreceptor CD8. This dimeric protein extends from the surface of a cytotoxic T cell and binds to class I MHC molecules that are expressed on the surface of the cell that is bound to the T cell. The dashed lines represent extended polypeptide chains that link the immunoglobulin domains of CD8 to the membrane. Notice that the coreceptor interacts primarily with the constant α3 domain of the class I MHC domain.
[Drawn from 1AKJ.pdb.]
On the basis of these components, a model for T-cell activation can be envisaged that is closely parallel to the pathway for B-cell activation (Section 34.3; Figure 34.33). The binding of the T-cell receptor to the class I MHC–peptide complex and the concomitant binding of CD8 from the T cell to the MHC molecule link the kinase Lck to the ITAM substrates of the components of the CD3 complex. The phosphorylation of the tyrosine residues in the ITAM sequences generates docking sites for a protein kinase called ZAP-70 (for 70-kDa zeta-associated protein) that is homologous to Syk in B cells. Docked by its two SH2 domains, ZAP-70 phosphorylates downstream targets in the signaling cascade. Additional molecules, including a membrane-bound protein phosphatase called CD45 and a cell-surface protein called CD28, play ancillary roles in this process.
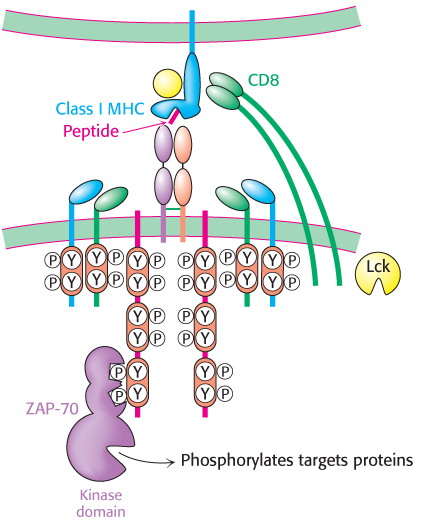
Figure 34.33: T-cell activation. The interaction between the T-cell receptor and a class I MHC–peptide complex results in the binding of CD8 to the MHC protein, the recruitment of the protein tyrosine kinase Lck, and the phosphorylation of tyrosine residues in the ITAM sequences of the CD3 chains. After phosphorylation, the ITAM regions serve as docking sites for the protein kinase ZAP-70, which phosphorylates protein targets to transmit the signal.
T-cell activation has two important consequences. First, the activation of cytotoxic T cells results in the secretion of several proteins, including perforin and granzymes. Perforin is a 70-kDa protein that destabilizes the plasma membrane of the target cell, enabling the entry of granzymes into the cytoplasm of the target cell. Granzymes are serine proteases (Section 9.1) that initiate the pathway of apoptosis, leading to the death of the target cell and the fragmentation of its DNA, including any viral DNA that may be present. Second, after it has stimulated its target cell to commit suicide, the activated T cell disengages and is stimulated to reproduce. Thus, additional T cells that express the same T-cell receptor are generated to continue the battle against the invader after these T cells have been identified as a suitable weapon.
Helper T cells stimulate cells that display foreign peptides bound to class II MHC proteins
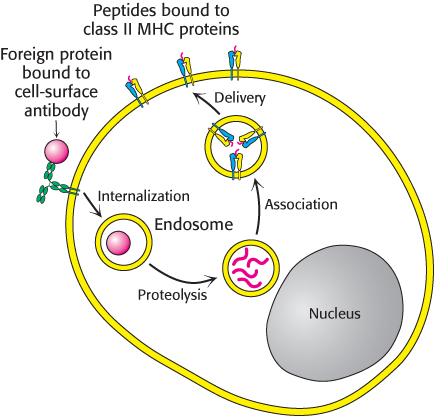
Figure 34.34: Presentation of peptides from internalized proteins. Antigen-presenting cells bind and internalize foreign proteins and display peptides that are formed from the digestion of these proteins in class II MHC proteins.
Not all T cells are cytotoxic. Helper T cells stimulate the proliferation of specific B lymphocytes and cytotoxic T cells and thereby serve as partners in determining the immune responses that are produced. The importance of helper T cells is graphically revealed by the devastation wrought by AIDS, a condition that destroys these cells. Helper T cells, like cytotoxic T cells, detect foreign peptides that are presented on cell surfaces by MHC proteins. However, the source of the peptides, the MHC proteins that bind them, and the transport pathway are different.
Helper T cells recognize peptides bound to MHC molecules referred to as class II. Their helping action is focused on B cells, macrophages, and dendritic cells. Class II MHC proteins are expressed only by these antigen-presenting cells, unlike class I MHC proteins, which are expressed on nearly all cells. The peptides presented by class II MHC proteins do not come from the cytoplasm. Rather, they arise from the degradation of proteins that have been internalized by endocytosis. Consider, for example, a virus particle that is captured by membrane-bound immunoglobulins on the surface of a B cell (Figure 34.34). This complex is delivered to an endosome, a membrane-enclosed acidic compartment, where it is digested. The resulting peptides become associated with class II MHC proteins, which move to the cell surface. Peptides from the cytoplasm cannot reach class II proteins, whereas peptides from endosomal compartments cannot reach class I proteins. This segregation of displayed peptides is biologically critical. The association of a foreign peptide with a class II MHC protein signals that a cell has encountered a pathogen and serves as a call for help. In contrast, association with a class I MHC protein signals that a cell has succumbed to a pathogen and is a call for destruction.
Helper T cells rely on the T-cell receptor and CD4 to recognize foreign peptides on antigen-presenting cells
The overall structure of a class II MHC molecule is remarkably similar to that of a class I molecule. Class II molecules consist of a 33-kDa α chain and a noncovalently bound 30-kDa β chain (Figure 34.35). Each contains two extracellular domains, a transmembrane segment, and a short cytoplasmic tail. The peptide-binding site is formed by the α1 and β1 domains, each of which contributes a long helix and part of a β sheet. Thus, the same structural elements are present in class I and class II MHC molecules, but they are combined into polypeptide chains in different ways. The peptide-binding site of a class II molecule is open at both ends, and so this groove can accommodate longer peptides than can be bound by class I molecules; typically, peptides between 13 and 18 residues long are bound. The peptide-binding specificity of each class II molecule depends on binding pockets that recognize particular amino acids, also known as anchor residues, in specific positions along the sequence.
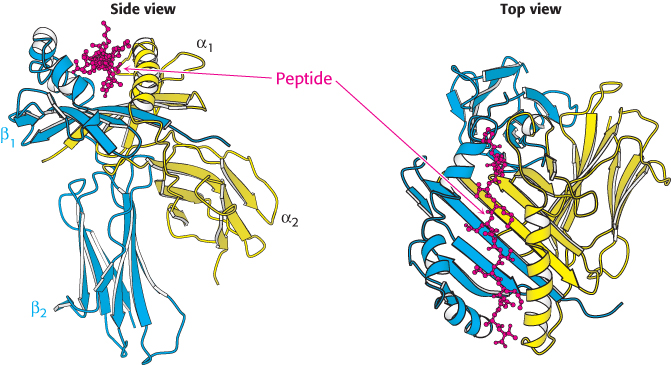
Figure 34.35:  Class II MHC protein. A class II MHC protein consists of homologous α and β chains, each of which has an amino-terminal domain that constitutes half of the peptide-binding structure, as well as a carboxyl-terminal immunoglobulin domain. Notice the trough-like peptide-binding site, which is similar to that in class I MHC proteins except that it is open at both ends, allowing class II MHC proteins to bind longer peptides than those bound by class I.
Class II MHC protein. A class II MHC protein consists of homologous α and β chains, each of which has an amino-terminal domain that constitutes half of the peptide-binding structure, as well as a carboxyl-terminal immunoglobulin domain. Notice the trough-like peptide-binding site, which is similar to that in class I MHC proteins except that it is open at both ends, allowing class II MHC proteins to bind longer peptides than those bound by class I.
[Drawn from 1DLH.pdb.]
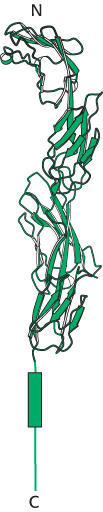
Figure 34.36:  Coreceptor CD4. This protein comprises four tandem immunoglobulin domains that extend from the surface of a helper T cell.
Coreceptor CD4. This protein comprises four tandem immunoglobulin domains that extend from the surface of a helper T cell.
[Drawn from 1WIO.pdb.]
Helper T cells express T-cell receptors that are produced from the same genes as those on cytotoxic T cells. These T-cell receptors interact with class II MHC molecules in a manner that is analogous to T-cell-receptor interaction with class I MHC molecules. Nonetheless, helper T cells and cytotoxic T cells are distinguished by other proteins that they express on their surfaces. In particular, helper T cells express a protein called CD4 instead of expressing CD8. CD4 consists of four immunoglobulin domains that extend from the T-cell surface, as well as a small cytoplasmic region (Figure 34.36). The amino-terminal immunoglobulin domains of CD4 interact with the base on the class II MHC molecule. Thus, helper T cells bind cells expressing class II MHC specifically because of the interactions with CD4 (Figure 34.37).
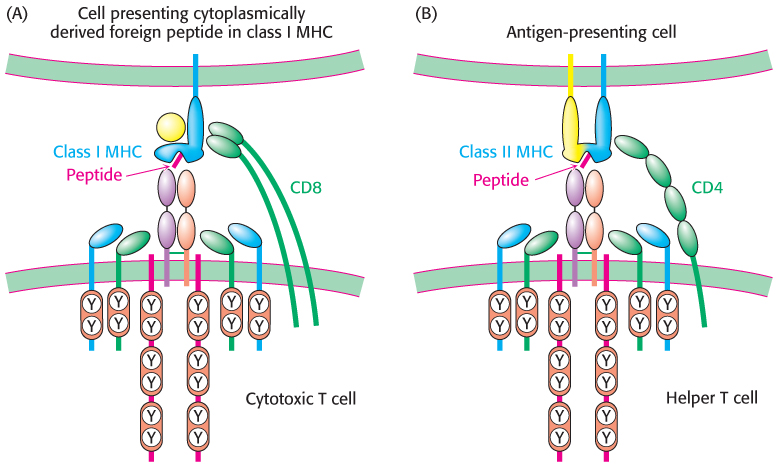
Figure 34.37: Variations on a theme. (A) Cytotoxic T cells recognize foreign peptides presented in class I MHC proteins with the aid of the coreceptor CD8. (B) Helper T cells recognize peptides presented in class II MHC proteins by specialized antigen-presenting cells with the aid of the coreceptor CD4.
When a helper T cell binds to an antigen-presenting cell expressing an appropriate class II MHC–peptide complex, signaling pathways analogous to those in cytotoxic T cells are initiated by the action of the kinase Lck on ITAMs in the CD3 molecules associated with the T-cell receptor. However, rather than triggering events leading to the death of the attached cell, these signaling pathways result in the secretion of cytokines from the helper cell. Cytokines are a family of molecules that include, among others, interleukin-2 and interferon-γ. Cytokines bind to specific receptors on the antigen-presenting cell and stimulate its growth and differentiation (Figure 34.38). For example, these cytokines stimulate the differentiation of B cells into plasma cells, which specialize in the production of large amounts of antibody. Thus, the internalization and presentation of parts of a foreign pathogen help to generate a local environment in which cells taking part in the defense against this pathogen can flourish through the action of helper T cells.
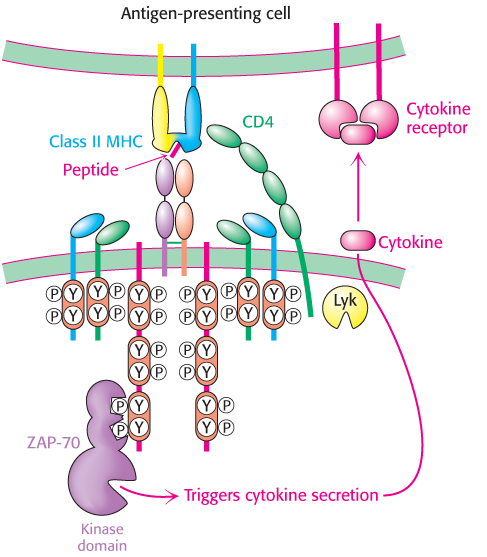
Figure 34.38: Helper-T-cell action. The engagement of the T-cell receptor in helper T cells results in the secretion of cytokines. These cytokines bind to cytokine receptors expressed on the surface of the antigen-presenting cell, stimulating cell growth, differentiation, and, in regard to a B cell, antibody secretion.
MHC proteins are highly diverse
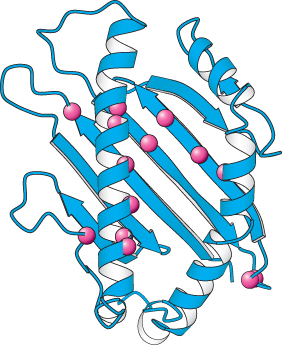
Figure 34.39:  Polymorphism in class I MHC proteins. Notice that the positions of sites with a high degree of polymorphism in the human population are displayed as red spheres on the structure of the amino-terminal part of a class I MHC protein.
Polymorphism in class I MHC proteins. Notice that the positions of sites with a high degree of polymorphism in the human population are displayed as red spheres on the structure of the amino-terminal part of a class I MHC protein.
[Drawn from 1HHK.pdb.]
 MHC class I and II proteins, the presenters of peptides to T cells, were discovered because of their role in transplantation rejection. A tissue transplanted from one person to another or from one mouse to another is usually rejected by the immune system. In contrast, tissues transplanted from one identical twin to another or between mice of an inbred strain are accepted. Genetic analyses revealed that rejection occurs when tissues are transplanted between individual organisms having different genes in the major histocompatibility complex, a cluster of more than 75 genes playing key roles in immunity. The 3500-kb span of the MHC is nearly the length of the entire E. coli chromosome. The MHC encodes class I proteins (presenters to cytotoxic T cells) and class II proteins (presenters to helper T cells), as well as class III proteins (components of the complement cascade) and many other proteins that play key roles in immunity.
MHC class I and II proteins, the presenters of peptides to T cells, were discovered because of their role in transplantation rejection. A tissue transplanted from one person to another or from one mouse to another is usually rejected by the immune system. In contrast, tissues transplanted from one identical twin to another or between mice of an inbred strain are accepted. Genetic analyses revealed that rejection occurs when tissues are transplanted between individual organisms having different genes in the major histocompatibility complex, a cluster of more than 75 genes playing key roles in immunity. The 3500-kb span of the MHC is nearly the length of the entire E. coli chromosome. The MHC encodes class I proteins (presenters to cytotoxic T cells) and class II proteins (presenters to helper T cells), as well as class III proteins (components of the complement cascade) and many other proteins that play key roles in immunity.
Human beings express six different class I genes (three from each parent) and six different class II genes. The three loci for class I genes are called HLA-A, -B, and -C; those for class II genes are called HLA-DP, -DQ, and -DR. These loci are highly polymorphic: many alleles of each are present in the population. For example, more than 50 each of HLA-A, -B, and -C alleles are known; the numbers discovered increase each year. Hence, the likelihood that two unrelated persons have identical class I and II proteins is very small (<10−4), accounting for transplantation rejection unless the genotypes of donor and acceptor are closely matched in advance.
Differences between class I proteins are located mainly in the α1 and α2 domains, which form the peptide-binding site (Figure 34.39). The α3 domain, which interacts with a constant β2-microglobulin, is largely conserved. Similarly, the differences between class II proteins cluster near the peptide-binding groove. Why are MHC proteins so highly variable? Their diversity makes the presentation of a very wide range of peptides to T cells possible. A particular class I or class II molecule may not be able to bind any of the peptide fragments of a viral protein. The likelihood of a fit is markedly increased by having several kinds (usually six) of each class of presenters in each individual organism. If all members of a species had identical class I or class II molecules, the population would be much more vulnerable to devastation by a pathogen that had mutated and thereby evaded presentation. The evolution of the diverse human MHC repertoire has been driven by the selection for individual members of the species who resist infections to which other members of the population may be susceptible.
Human immunodeficiency viruses subvert the immune system by destroying helper T cells
 In 1981, the first cases of a new disease now called acquired immune deficiency syndrome (AIDS) were recognized. The victims died of rare infections because their immune systems were crippled. The cause was identified 2 years later by Luc Montagnier and coworkers. AIDS is produced by human immunodeficiency virus (HIV), of which two major classes are known: HIV-1 and the much less common HIV-2. Like other retroviruses, HIV contains a single-stranded RNA genome that is replicated through a double-stranded DNA intermediate. This viral DNA becomes integrated into the genome of the host cell. In fact, viral genes are transcribed only when they are integrated into the host DNA.
In 1981, the first cases of a new disease now called acquired immune deficiency syndrome (AIDS) were recognized. The victims died of rare infections because their immune systems were crippled. The cause was identified 2 years later by Luc Montagnier and coworkers. AIDS is produced by human immunodeficiency virus (HIV), of which two major classes are known: HIV-1 and the much less common HIV-2. Like other retroviruses, HIV contains a single-stranded RNA genome that is replicated through a double-stranded DNA intermediate. This viral DNA becomes integrated into the genome of the host cell. In fact, viral genes are transcribed only when they are integrated into the host DNA.
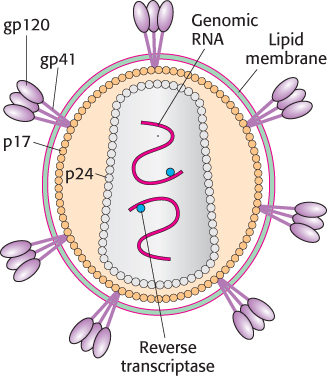
Figure 34.40: Human immunodeficiency virus. A schematic representation of HIV reveals its proteins and nucleic acid components.
[Information from G. B. Karlsson Hedestam et al., Nat. Rev. Microbiol. 6:143–155, 2008, Fig. 2a.]
The HIV virion is enveloped by a lipid-bilayer membrane containing two glycoproteins: gp41 spans the membrane and is associated with gp120, which is located on the external face (Figure 34.40). The core of the virus contains two copies of the RNA genome and associated transfer RNAs, as well as several molecules of reverse transcriptase. They are surrounded by many copies of two proteins called p17 and p24. The host cell for HIV is the helper T cell. The gp120 molecules on the membrane of HIV bind to CD4 molecules on the surface of the helper T cell (Figure 34.41). This interaction allows the associated viral gp41 to insert its amino-terminal head into the host-cell membrane. The viral membrane and the helper-T-cell membrane fuse, and the viral core is released directly into the cytoplasm. Infection by HIV leads to the destruction of helper T cells because the permeability of the host plasma membrane is markedly increased by the insertion of viral glycoproteins and the budding of virus particles. The influx of ions and water disrupts the ionic balance, causing osmotic lysis.
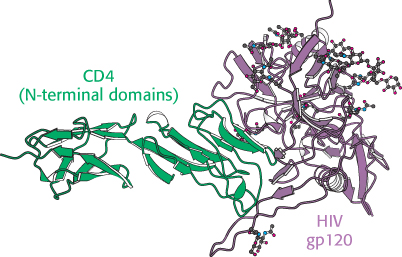
Figure 34.41:  HIV receptor. A complex between a modified form of the envelope glycoprotein gp120 from HIV and a peptide corresponding to the two amino-terminal domains from the helper-T-cell protein CD4 reveals how viral infection of helper T cells is initiated.
HIV receptor. A complex between a modified form of the envelope glycoprotein gp120 from HIV and a peptide corresponding to the two amino-terminal domains from the helper-T-cell protein CD4 reveals how viral infection of helper T cells is initiated.
[Drawn from 1GC1.pdb.]



 Class I MHC protein. A protein of this class consists of two chains. Notice that the α chain begins with two domains (α1, α2) that include α helices and continues with an immunoglobulin domain (α3), a transmembrane domain, and a cytoplasmic tail. The second chain, β2-microglobulin, adopts an immunoglobulin fold.
Class I MHC protein. A protein of this class consists of two chains. Notice that the α chain begins with two domains (α1, α2) that include α helices and continues with an immunoglobulin domain (α3), a transmembrane domain, and a cytoplasmic tail. The second chain, β2-microglobulin, adopts an immunoglobulin fold.

 Class I MHC peptide-
Class I MHC peptide-

 T-
T-
 T-
T-

 The coreceptor CD8. This dimeric protein extends from the surface of a cytotoxic T cell and binds to class I MHC molecules that are expressed on the surface of the cell that is bound to the T cell. The dashed lines represent extended polypeptide chains that link the immunoglobulin domains of CD8 to the membrane. Notice that the coreceptor interacts primarily with the constant α3 domain of the class I MHC domain.
The coreceptor CD8. This dimeric protein extends from the surface of a cytotoxic T cell and binds to class I MHC molecules that are expressed on the surface of the cell that is bound to the T cell. The dashed lines represent extended polypeptide chains that link the immunoglobulin domains of CD8 to the membrane. Notice that the coreceptor interacts primarily with the constant α3 domain of the class I MHC domain.



 Class II MHC protein. A class II MHC protein consists of homologous α and β chains, each of which has an amino-
Class II MHC protein. A class II MHC protein consists of homologous α and β chains, each of which has an amino-
 Coreceptor CD4. This protein comprises four tandem immunoglobulin domains that extend from the surface of a helper T cell.
Coreceptor CD4. This protein comprises four tandem immunoglobulin domains that extend from the surface of a helper T cell.



 Polymorphism in class I MHC proteins. Notice that the positions of sites with a high degree of polymorphism in the human population are displayed as red spheres on the structure of the amino-
Polymorphism in class I MHC proteins. Notice that the positions of sites with a high degree of polymorphism in the human population are displayed as red spheres on the structure of the amino- MHC class I and II proteins, the presenters of peptides to T cells, were discovered because of their role in transplantation rejection. A tissue transplanted from one person to another or from one mouse to another is usually rejected by the immune system. In contrast, tissues transplanted from one identical twin to another or between mice of an inbred strain are accepted. Genetic analyses revealed that rejection occurs when tissues are transplanted between individual organisms having different genes in the major histocompatibility complex, a cluster of more than 75 genes playing key roles in immunity. The 3500-
MHC class I and II proteins, the presenters of peptides to T cells, were discovered because of their role in transplantation rejection. A tissue transplanted from one person to another or from one mouse to another is usually rejected by the immune system. In contrast, tissues transplanted from one identical twin to another or between mice of an inbred strain are accepted. Genetic analyses revealed that rejection occurs when tissues are transplanted between individual organisms having different genes in the major histocompatibility complex, a cluster of more than 75 genes playing key roles in immunity. The 3500- In 1981, the first cases of a new disease now called acquired immune deficiency syndrome (AIDS) were recognized. The victims died of rare infections because their immune systems were crippled. The cause was identified 2 years later by Luc Montagnier and coworkers. AIDS is produced by human immunodeficiency virus (HIV), of which two major classes are known: HIV-
In 1981, the first cases of a new disease now called acquired immune deficiency syndrome (AIDS) were recognized. The victims died of rare infections because their immune systems were crippled. The cause was identified 2 years later by Luc Montagnier and coworkers. AIDS is produced by human immunodeficiency virus (HIV), of which two major classes are known: HIV-

 HIV receptor. A complex between a modified form of the envelope glycoprotein gp120 from HIV and a peptide corresponding to the two amino-
HIV receptor. A complex between a modified form of the envelope glycoprotein gp120 from HIV and a peptide corresponding to the two amino-