35.4 A Rotary Motor Drives Bacterial Motion
In 1 s, a motile bacterium can move approximately 25 μm, or about 10 body lengths. A human being sprinting at a proportional rate would complete the 100-meter dash in slightly more than 5 s. The motors that power this impressive motion are strikingly different from the eukaryotic motors that we have seen so far. In the bacterial motor, an element spins around a central axis rather than moving along a polymeric track. The direction of rotation can change rapidly, a feature that is central to chemotaxis, the process by which bacteria swim preferentially toward an increasing concentration of certain useful compounds and away from potentially harmful ones. One type of flagellar motor, powered by a Na+ gradient, turns at a rate of 200,000 revolutions per minute.
Bacteria swim by rotating their flagella
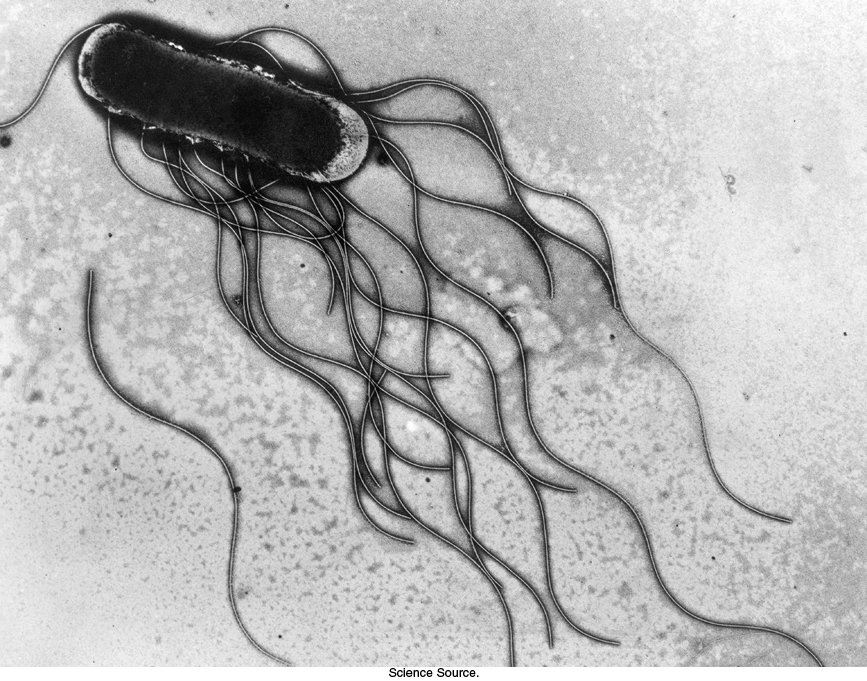
Figure 35.25: Bacterial flagella. Electron micrograph of Salmonella typhimurium shows flagella directed backwards, where they can form a bundle under appropriate circumstances.
[Science Source.]
Bacteria such as Escherichia coli and Salmonella typhimurium swim by rotating flagella that lie on their surfaces (Figure 35.25). When the flagella rotate in a counterclockwise direction (viewed from outside a bacterium), the separate flagella form a bundle that very efficiently propels the bacterium through solution.
Bacterial flagella are polymers approximately 15 nm in diameter and as much as 15 μm in length, composed of 53-kDa subunits of a protein called flagellin (Figure 35.26). These subunits associate into a helical structure that has 5.5 subunits per turn, giving the appearance of 11 protofilaments. Each flagellum has a hollow core. Remarkably, flagella form not by growing at the base adjacent to the cell body but, instead, by the addition of new subunits that pass through the hollow core and add to the free end. Each flagellum is intrinsically twisted in a left-handed sense. At its base, each flagellum has a rotory motor.
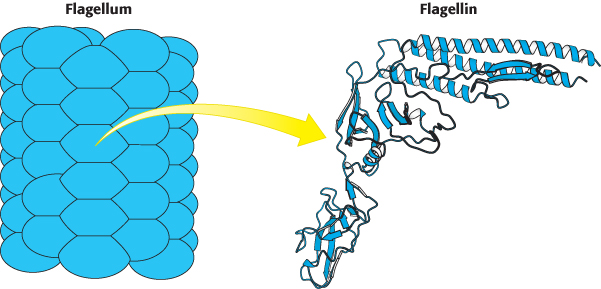
Figure 35.26:  Structure of flagellin. A bacterial flagellum is a helical polymer of the protein flagellin. Notice that each subunit corresponds to a bent structure with a relatively flat surface facing the hollow core of the flagellum.
Structure of flagellin. A bacterial flagellum is a helical polymer of the protein flagellin. Notice that each subunit corresponds to a bent structure with a relatively flat surface facing the hollow core of the flagellum.
[Drawn from 1IO1.pdb.]
Proton flow drives bacterial flagellar rotation
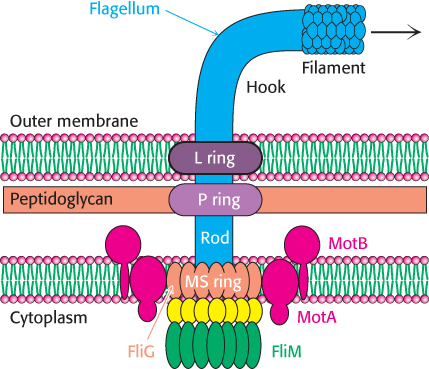
Figure 35.27: Flagellar motor. A schematic view of the flagellar motor, a complex structure containing as many as 40 distinct types of protein. The approximate positions of the proteins MotA and MotB (red), FliG (orange), FliN (yellow), and FliM (green) are shown.
Early experiments by Julius Adler demonstrated that ATP is not required for flagellar motion. What powers these rotary motors? The necessary free energy is derived from the proton gradient that exists across the plasma membrane. The flagellar motor is quite complex, containing as many as 40 distinct proteins (Figure 35.27). Five components particularly crucial to motor function have been identified through genetic studies. MotA is a membrane protein that appears to have four transmembrane helices as well as a cytoplasmic domain. MotB is another membrane protein with a single trans-membrane helix and a large periplasmic domain. Approximately 11 MotA–MotB pairs form a ring around the base of the flagellum. The proteins FliG, FliM, and FliN are part of a disc-like structure called the MS (membrane and supramembrane) ring, with approximately 30 FliG subunits coming together to form the ring. The three-dimensional structure of the carboxyl-terminal half of FliG reveals a wedge-shaped domain with a set of charged amino acids, conserved among many species, lying along the thick edge of the wedge (Figure 35.28).
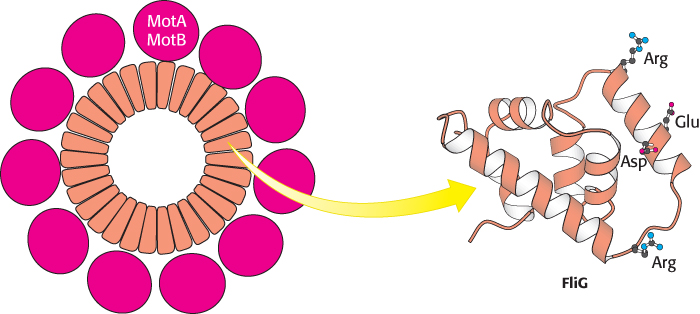
Figure 35.28:  Flagellar motor components. Approximately 30 subunits of FliG assemble to form part of the MS ring. The ring is surrounded by approximately 11 structures consisting of MotA and MotB. Notice that the carboxyl-terminal domain of FliG includes a ridge lined with charged residues that may participate in proton transport.
Flagellar motor components. Approximately 30 subunits of FliG assemble to form part of the MS ring. The ring is surrounded by approximately 11 structures consisting of MotA and MotB. Notice that the carboxyl-terminal domain of FliG includes a ridge lined with charged residues that may participate in proton transport.
[Drawn from 1QC7.pdb.]
The MotA–MotB pair and FliG combine to create a proton channel that drives the rotation of the flagellum. How can proton flow across a membrane drive mechanical rotation? We have seen such a process earlier in regard to ATP synthase (Section 18.4). Recall that the key to driving the rotation of the γ subunit of ATP synthase is the a subunit of the F0 fragment. This subunit appears to have two half-channels; protons can move across the membrane only by moving into the half-channel from the side of the membrane with the higher local proton concentration, binding to a disc-like structure formed by the c subunits, riding on this structure as it rotates to the opening of the other half-channel, and exiting to the side with the lower local proton concentration. Could a similar mechanism apply to flagellar rotation? Indeed, such a mechanism was first proposed by Howard Berg to explain flagellar rotation before the rotary mechanism of ATP synthase was elucidated. Each MotA–MotB pair is conjectured to form a structure that has two half-channels; FliG serves as the rotating proton carrier, perhaps with the participation of some of the charged residues identified in crystallographic studies (Figure 35.29). In this scenario, a proton from the periplasmic space passes into the outer half-channel and is transferred to an FliG subunit. The MS ring rotates, rotating the flagellum with it and allowing the proton to pass into the inner half-channel and into the cell. Ongoing structural and mutagenesis studies are testing and refining this hypothesis.
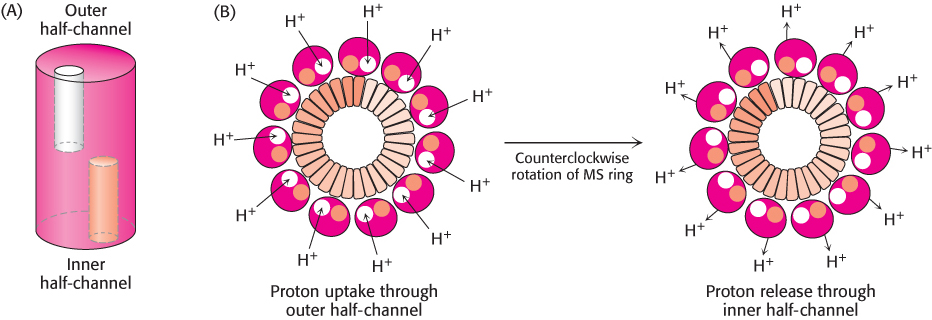
Figure 35.29: Proton-transport-coupled rotation of the flagellum. (A) MotA–MotB may form a structure having two half-channels. (B) One model for the mechanism of coupling rotation to a proton gradient requires protons to be taken up into the outer half-channel and transferred to the MS ring. The MS ring rotates in a counterclockwise direction, and the protons are released into the inner half-channel. The flagellum is linked to the MS ring and so the flagellum rotates as well.
Bacterial chemotaxis depends on reversal of the direction of flagellar rotation
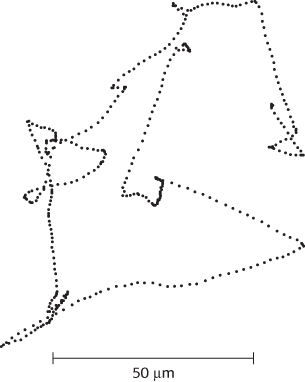
Figure 35.30: Charting a course. This projection of the track of an E. coli bacterium was obtained with a microscope that automatically follows bacterial motion in three dimensions. The points show the locations of the bacterium at 80-ms intervals.
[Information from H. C. Berg, Nature 254:389–392, 1975.]
Many species of bacteria respond to changes in their environments by adjusting their swimming behavior. Examination of the paths taken is highly revealing (Figure 35.30). The bacteria swim in one direction for some length of time (typically about a second), tumble briefly, and then set off in a new direction. The tumbling is caused by a brief reversal in the direction of the flagellar motor. When the flagella rotate counterclockwise, the helical filaments form a coherent bundle favored by the intrinsic shape of each filament, and the bacterium swims smoothly. When the rotation reverses, the bundle flies apart because the screw sense of the helical flagella does not match the direction of rotation. Each flagellum then pulls in a different direction and the cell tumbles.
In the presence of a gradient of certain substances such as glucose, bacteria swim preferentially toward the direction of the higher concentration of the substance. Such compounds are referred to as chemoattractants. Bacteria also swim preferentially away from potentially harmful compounds such as phenol, a chemorepellant. The process of moving in specific directions in response to environmental cues is called chemotaxis. In the presence of a gradient of a chemoattractant, bacteria swim for longer periods of time without tumbling when moving toward higher concentrations of the chemoattractant. In contrast, they tumble more frequently when moving toward lower concentrations of the chemoattractant. This behavior is reversed for chemorepellants. The result of these actions is a biased random walk that facilitates net motion toward conditions more favorable to the bacterium.
Chemotaxis depends on a signaling pathway that terminates at the flagellar motor. The signaling pathway begins with the binding of molecules to receptors in the plasma membrane (Figure 35.31). In their unoccupied forms, these receptors initiate a pathway leading eventually to the phosphorylation of a specific aspartate residue on a soluble protein called CheY. In its phosphorylated form, CheY binds to the base on the flagellar motor. When bound to phosphorylated CheY, the flagellar motor rotates in a clockwise rather than a counterclockwise direction, causing tumbling.
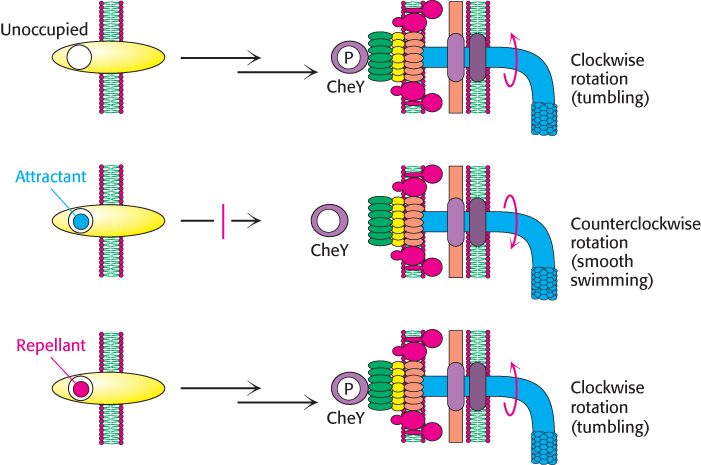
Figure 35.31: Chemotaxis signaling pathway. Receptors in the plasma membrane initiate a signaling pathway leading to the phosphorylation of the CheY protein. Phosphorylated CheY binds to the flagellar motor and favors clockwise rotation. When an attractant binds to the receptor, this pathway is blocked, and counterclockwise flagellar rotation and, hence, smooth swimming result. When a repellant binds, the pathway is stimulated, leading to an increased concentration of phosphorylated CheY and, hence, more-frequent clockwise rotation and tumbling.
The binding of a chemoattractant to a surface receptor blocks the signaling pathway leading to CheY phosphorylation. Phosphorylated CheY spontaneously hydrolyzes and releases its phosphoryl group in a process accelerated by another protein, CheZ. The concentration of phosphorylated CheY drops, and the flagella are less likely to rotate in a clockwise direction. Under these conditions, bacteria swim smoothly without tumbling. Thus, the reversible rotary flagellar motor and a phosphorylation-based signaling pathway work together to generate an effective means for responding to environmental conditions.
Bacteria sense spatial gradients of chemoattractants by measurements separated in time. A bacterium sets off in a random direction and, if the concentration of the chemoattractant has increased after the bacterium has been swimming for a period of time, the likelihood of tumbling decreases and the bacterium continues in roughly the same direction. If the concentration has decreased, the tumbling frequency increases and the bacterium tests other random directions. The success of this mechanism once again reveals the power of evolutionary problem solving: many possible solutions are tried at random, and those that are beneficial are selected and exploited.


 Structure of flagellin. A bacterial flagellum is a helical polymer of the protein flagellin. Notice that each subunit corresponds to a bent structure with a relatively flat surface facing the hollow core of the flagellum.
Structure of flagellin. A bacterial flagellum is a helical polymer of the protein flagellin. Notice that each subunit corresponds to a bent structure with a relatively flat surface facing the hollow core of the flagellum.


 Flagellar motor components. Approximately 30 subunits of FliG assemble to form part of the MS ring. The ring is surrounded by approximately 11 structures consisting of MotA and MotB. Notice that the carboxyl-
Flagellar motor components. Approximately 30 subunits of FliG assemble to form part of the MS ring. The ring is surrounded by approximately 11 structures consisting of MotA and MotB. Notice that the carboxyl-

