From Genotype to Phenotype
As already explained, when a sperm and ovum create a zygote, they establish the genotype: all the genes of the developing person. That begins several complex processes that combine to form the phenotype—the person’s appearance, behavior, and brain and body functions. Nothing is totally genetic, not even such obvious traits as height or hair color, but nothing is untouched by genes, including working overtime or not at all, wanting or refusing a divorce, or becoming a devoted or a rejecting parent (Plomin et al., 2013).
80
The genotype instigates body and brain formation, but the phenotype depends on many genes and on the environment. The phenotype is influenced from the moment of conception until the moment of death, sometimes directly (epigenetic) and sometime via cultural and familial circumstances.
Almost every trait is polygenic (affected by many genes) and multifactorial (influenced by many factors). A zygote might have the alleles for becoming, say, a musical genius, but that potential may never be expressed. Accurate prediction of the phenotype is impossible, even if the genotype is entirely known (Lehner, 2013).
Almost daily, researchers describe additional complexities in polygenic and multifactorial interaction. It is apparent that “phenotypic variation … results from multiple interactions among numerous genetic and environmental factors.” To describe this “fundamental problem of interrelating genotype and phenotype in complex traits” (Nadeau & Dudley, 2011, p. 1015), we begin with epigenetics.
Epigenetics

All important human characteristics are epigenetic; this applies also to diseases that are known to be inherited, such as cancer, schizophrenia, and autism (Kundu, 2013; Plomin et al., 2013).
Type 2 diabetes is a notable example. People who inherit genes that put them at risk do not always become diabetic. However, lifestyle factors might activate that genetic risk. If that happens, epigenetic changes to the genes make diabetes irreversible: Diet and insulin may control the disease, but the pre-
One intervention—
The same may be true for other developmental changes over the life span. Drug use—
In general, environmental influences (such as injury, temperature extremes, drug abuse, and crowding) can impede healthy development, whereas others (nourishing food, loving care, play) can facilitate it, all because of differential susceptibility and epigenetic change. A recent discovery is that some environmental factors that suppress or release genes are cognitive, not biological.
For example, if a person feels lonely and rejected, that feeling can affect the RNA, which allows genetic potential for heart disease or social anxiety to be expressed (Slavich & Cole, 2013). Note that the feeling of loneliness, not the objective number of friends or social contacts, has significant epigenetic influence.
OBSERVATION QUIZ Who has that genetic condition?
Ellie has a gene for achondroplasia, the most common form of dwarfism, which affects her limb growth, making her a little person. Because of her parents and her sister, she is likely to have a long and accomplished life: Her problems are less likely to come from her genotype than from how other people perceive her phenotype.
No trait—
81
A surprising example might be political ideology: Several researchers report that a particular allele of a dopamine receptor gene (DRD4-
Gene–Gene Interactions
Many discoveries have followed the completion of the Human Genome Project in 2001. One of the first surprises was that humans have far fewer than 100,000 genes, the number often cited in the twentieth century. A person has a total of about 20,000 genes.
The precise number is elusive because—
Additive Heredity
Some genes and alleles are additive because their effects add up to influence the phenotype. When genes interact additively, the phenotype usually reflects the contributions of every gene that is involved. Height, hair curliness, and skin color, for instance, are usually the result of additive genes. Indeed, height is probably influenced by 180 genes, each contributing a very small amount (Enserink, 2011).
Most people have ancestors of varied height, hair curliness, skin color, and so on, so their children’s phenotype does not mirror the parents’ phenotypes (although the phenotype always reflects the genotype). I see this in my family: Our daughter Rachel is of average height, shorter than her father or me, but taller than either of our mothers. She apparently inherited some of her grandmothers’ height genes via our gametes. And none of my children have exactly my skin color—

Especially for Future Parents Suppose you wanted your daughters to be short and your sons to be tall. Could you achieve that?
Possibly, but you wouldn’t want to. You would have to choose one mate for your sons and another for your daughters, and you would have to use sex-
82
How any additive trait turns out depends partly on all the genes a child happens to inherit (half from each parent, which means one-
Dominant–Recessive Heredity
Not all genes are additive. In one non-
Everyone has recessive genes that are not apparent in the phenotype. For instance, no one would guess, simply by looking at me, that my mother had to stretch to reach 5’4.” But Rachel has half of her genes from me, and one of my height genes may be recessive. Every person is a carrier of recessive genes, carried on the genotype but not expressed in the phenotype.
Most recessive genes are harmless. For example, blue eyes are determined by a recessive allele and brown eyes by a dominant one, so a child conceived by a blue-
“Usually have brown eyes” but not always. Sometimes a brown-
This gets tricky if both parents are carriers. Thus if two brown-
OBSERVATION QUIZ Why do these four offspring look identical except for eye color?
This is a figure, drawn to illustrate the recessive inheritance of blue eyes, and thus eyes are the only difference shown. If this were a real family, each child would have a distinct appearance.
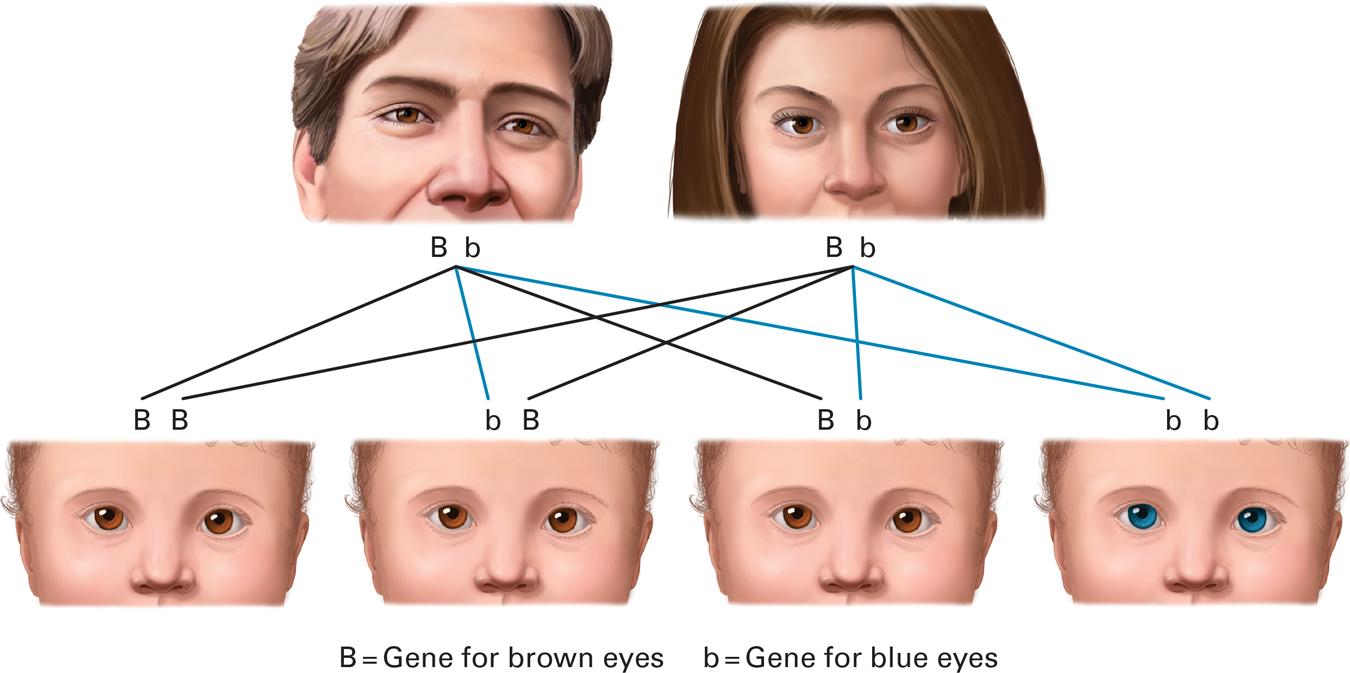
Changeling? No. If two brown-
83
The crucial fact is that a recessive gene is carried, but not apparent, unless a child happens to inherit the same recessive gene from both parents. Most recessive genes are harmless (like the genes for blue eyes) but some are lethal (like those that cause serious diseases, discussed at the end of this chapter). It is important to understand the double recessive, so parents do not blame each other when their child has a recessive disease, and husbands do not suspect infidelity if his child is unexpectedly blue-
A special case of the dominant–
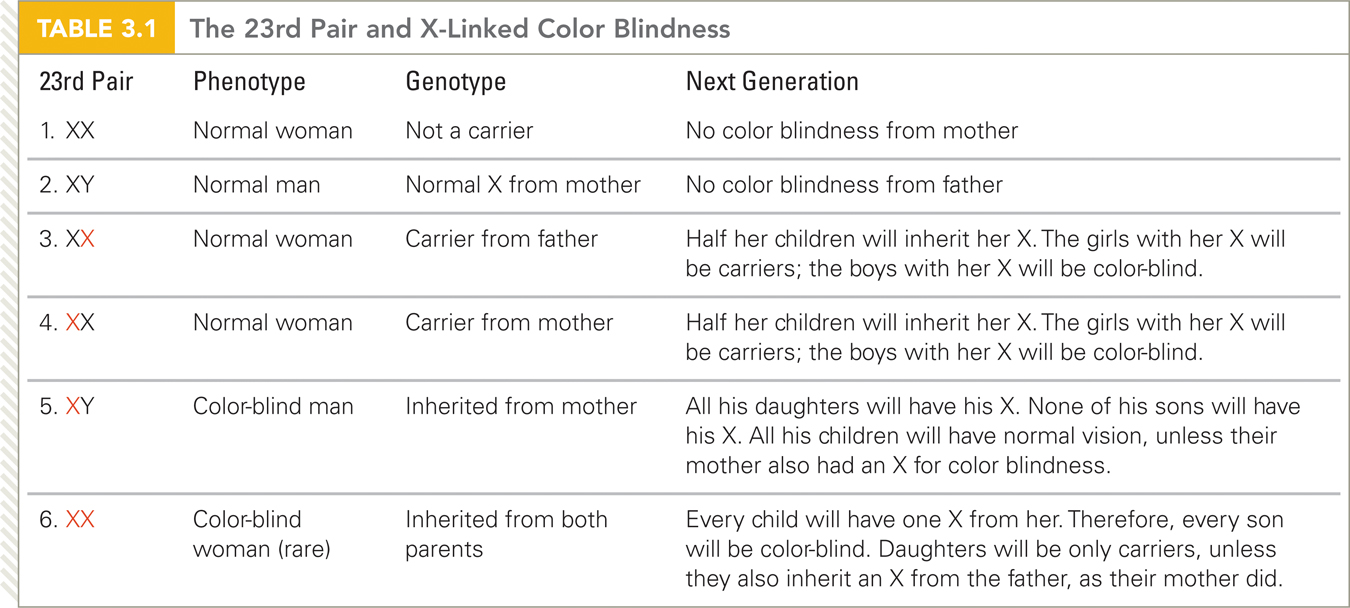
To understand this, remember that the Y chromosome is much smaller than the X, containing far fewer genes. For that reason, genes on the X almost never have a match on the Y. Therefore, recessive traits carried on the X have no dominant gene on the Y.
A boy, XY, with a recessive gene on his X, has no gene on his Y to counteract it, so his phenotype is affected. A girl will be affected only if she has the recessive trait on both her X chromosomes, which is rare. This explains why males with an X-
Copy Number Variations
For any living creature, the outcomes of all the interactions involved in heredity are difficult to predict. A small deletion, repetition, or transposition in any of the 3 billion base pairs may be inconsequential, deadly, or something in between.
When the human genome was first mapped in 2001, it was hoped that a specific additive, recessive, or dominant gene could be located for each genetic disorder. Then a cure would soon follow. That “one gene/one disorder” expectation proved to be fantasy, disappointing many doctors who hoped that personalized medicine was imminent (Marshall, 2011). Molecular analysis found, instead, that thousands of seemingly minor variations in base pairs turn out to be influential—
84
Attention has focused on copy number variations, which are genes with repeats (from one to hundreds) or deletions of base pairs. Copy number variations correlate with almost every disease and condition, including heart disease, impaired intellectual abilities, mental illness, and many cancers.
We all have copy number variations: There is no person who is completely normal, genetically. One team, looking specifically at genes for neurons in the brain, says “dysfunctions abound … with a large number of functional variants in every genome” (Macosko & McCarroll, 2013, p. 564).
Especially For Medical Doctors Can you look at a person and then write a prescription that will personalize medicine to their particular genetic susceptibility?
No. Personalized medicine is the hope of many physicians, but appearance (the phenotype) does not indicate alleles, recessive genes, copy number variations, and other genetic factors that affect drug reactions. Many medical researchers seek to personalize chemotherapy for cancer, but although this is urgently needed, success is still experimental, even when the genotype is known.
Researchers are just beginning to understand the implications of copy number variations, although many hope that soon such genetic information will help target drugs and other medical measures, such as which cocktail of chemotherapy will stop which cancer. Even that may be a hopeful fantasy. Since epigenetics shows that environmental influences can actually change genetic expression, personalized medicine must consider each individual’s habits of mind and life at least as much as their genes (Horwitz et al., 2013).
To further complicate matters, sometimes one-
Parental Imprinting
X deactivation in girls but not boys is not the only case that is affected by a person’s sex. Sometimes the same allele affects male and female embryos differently. It also matters whether a gene came from the mother or the father, a phenomenon called parental imprinting.
The best-

85
Parental imprinting is quite common. Early in prenatal development (day 15), an estimated 553 genes act differently depending on whether they come from the mother or from the father—
SUMMING UP The distinction between genotype (heredity) and phenotype (manifest appearance and observed behavior) is one of the many complexities in genetics and human development. All traits are epigenetic, the product of genetic and nongenetic influences, beginning with methylation at conception and continuing lifelong. Furthermore, most traits are polygenic, the result of many genes that interact—
WHAT HAVE YOU LEARNED?
Question 3.12
wNc7KXkpVQZ+pX1BcO4m55KSzKiof7NZVNYGe4dYRJMBoMCeJFbERdDP3jK6w77EMEhQ5GwQHfcqO+xkMFN9yQ==The genotype underlies a person' s body and brain formation, but the person's phenotype ( the visible traits and behaviors) depends on many genes and on the environment.Question 3.13
wNZ3JGcPqMk72vY39nfY9jwaAi/NDz607jhkslc8UCf8qJ93vQgjiNFR8N5paTxQmyF9ItTvuCpl8pjA9VznRvoGvGQjXBFIFYJmPsPVwQLQFwHYSIXKf0ogicM=Epigenetic characteristics are those for which environmental factors affect genes and their expression. A multifactorial trait is a trait that is affected by many factors, both genetic and environmental. In both cases, the expression of genes is enhanced, halted, shaped, or altered, resulting in a phenotype that may differ markedly from the genotype.Question 3.14
2Gaw7yvxl0UVVOOe+DEMDjFUMQ4WptLIF8jOqvRtramxj91nSKBCPM6BwY+2yT8hOwFt2r/WfvY=The main patterns of genetic interaction include additive heredity, dominant–recessive heredity, copy number variations, and parental imprinting. Question 3.15
wosQ82skhaf9kymlbzUUb34g3Y0A3KGhKqq5Ma05F0rWs6zkaoWffrqRu7DlNodEXhPaEtw9BeO1OrOxNPh68oTJPvTF5PYC/0xjo4YPMdjM4loNUf6Fns8kkqht5+f5ANb4S/gw9vZUkmWrQOhzEB5YHnQKlbVHPR/vdrnUWeqkEACxQJB4/5YN/oL1pzCKfDnS2rUiwz0=Polygenic traits are traits in the phenotype that are determined by many genes. One example is height, which is determined by the interaction of approximately 180 different genes acting together, all contributing a bit to the expression of the trait; these genes act additively. Hair curliness and skin color are also the result of additive genes. In the case of dominant–recessive genes, the expression of a trait in the phenotype depends on just one gene pair. An example is eye color. The brown– eye gene is dominant and the blue– eye gene is recessive. The eye color of a child depends solely on the action of these two genes: a child who inherits two brown– eye genes or a brown– eye gene and a blue– eye gene will have brown eyes, whereas a child who inherits two blue– eye genes will have blue eyes. The sheer number of additive genes that can contribute to a single trait suggests that additive genes are more common than dominant– recessive ones. Question 3.16
d9YG5bJrXlwfJcjtCmed0orJUthwo5iSDJsxj/4OOM2aLvyeOT+W3W7yH85camdAnlq26rytdITtW9RhnE2Om/UZgsg=The best–known example of parental imprinting occurs with a small deletion on chromosome 15. If that deletion came from the father' s chromosome 15, the child may develop Prader– Willi syndrome and be obese, slow– moving, and stubborn. If that deletion came from the mother' s chromosome 15, the child will have Angelman syndrome and be thin, hyperactive, and happy– sometimes too happy, laughing when no one else does. In both cases, intellectual development is impaired, though in somewhat distinct ways.