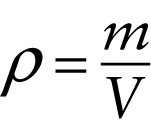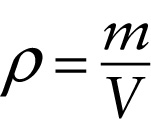Definition of density (11-1)
Question 1 of 3
Question
...equals the mass \(m\) of a given quantity of that substance...
{"title":"The density rho of a certain substance","description":"Wrong","type":"incorrect","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"2,44,47,100\"}]"} {"title":"...equals the mass m of a given quantity of that substance...","description":"Correct!","type":"correct","color":"#ffcc00","code":"[{\"shape\":\"rect\",\"coords\":\"118,11,119,13\"},{\"shape\":\"rect\",\"coords\":\"101,22,150,54\"}]"} {"title":"...divided by the volume V of that quantity of the substance.","description":"Incorrect","type":"incorrect","color":"#333300","code":"[{\"shape\":\"poly\",\"coords\":\"113,132\"},{\"shape\":\"rect\",\"coords\":\"195,33,199,34\"},{\"shape\":\"poly\",\"coords\":\"132,99\"},{\"shape\":\"rect\",\"coords\":\"105,73,148,118\"}]"}Review
One way to describe how tightly packed the molecules are in a gas, liquid, or solid is in terms of \(\textit{density}\). The \(\textbf{density}\) of a substance, denoted by the Greek letter \(\rho\) (rho), is the mass of the substance divided by the volume that it occupies. The greater the density of a substance, the more kilograms of that substance are packed into a given number of cubic meters of volume: