Chapter 15. Molar specific heat at constant volume for an ideal gas (15-18)
Question
uZU9CQ7UbJCXP/Y5V1DXW7RYbBL2y4uVJGQrztr160Nm0QrXyCsxS/2T/lsus7agel5G2mf2H9BmYh8z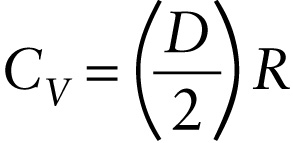
Question
Ga8PI+cWuZ0n7BavcLHkD451gPDVonmPqrXUrDazZbiwvM//+Zi3vLPcRd4OdC/61FAKZC4xQtTMLDMJ+j9+pA==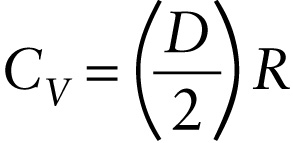
Question
wtRAiJSdJqY9BBQst0hZPa7Zvo2EBLDaMFekFg==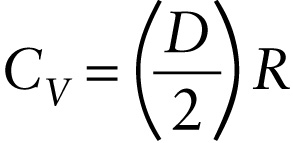
Review
We'll use the symbol \(D\) to represent the number of degress of freedom. Then the average energy per gas molecule is \((D/2)kT\). One mole of the gas contains Avogadro's number of molecules, so the energy per mole is \(N_A(D/2)kT = (D/2)\ (N_Ak)T = (D/2)RT\). (Again, recall that \(k = R/N_A\), so \(R = N_Ak)\).) Then the internal energy of \(n\) moles of ideal gas is
(15-16) \(U = n\left(\frac{D}{2}R\right)T\) (ideal gas, \(D\) degrees of freedom)
If the temperature of the gas changes by \(\Delta{T}\), it follows from Equation 15-16 that the internal energy change is
(15-17) \(\Delta{U} = n\left(\frac{D}{2}R\right)\Delta{T}\) (ideal gas, \(D\) degrees of freedom)
But, we saw above that for an ideal gas, \(\Delta{U} = nC_V\ \Delta{T}\). Comparing this to Equation 15-17, we see that
