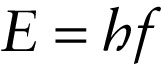Energy of a photon (22-26)
Question 1 of 3
Question
Energy of a photon
{"title":"Energy of a photon","description":"Correct!","type":"correct","color":"#99CCFF","code":"[{\"shape\":\"poly\",\"coords\":\"82,133\"},{\"shape\":\"rect\",\"coords\":\"10,16,12,16\"},{\"shape\":\"poly\",\"coords\":\"144,22\"},{\"shape\":\"rect\",\"coords\":\"3,8,40,54\"}]"} {"title":"Wave frequency","description":"Incorrect","type":"incorrect","color":"#008000","code":"[{\"shape\":\"rect\",\"coords\":\"134,7,159,65\"}]"} {"title":"Planck’s constant = 6.62606957 × 10–34 J • s","description":"Incorrect","type":"incorrect","color":"#333300","code":"[{\"shape\":\"rect\",\"coords\":\"101,6,132,56\"}]"}Review
The energy of an electromagnetic wave propagates as small, individual packets of energy called photons. The energy of an individual photon is proportional to the wave frequency, and the proportionality constant h is called Planck’s constant:
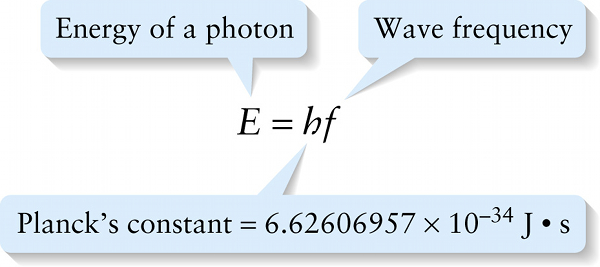
To three significant figures, \(h = 6.63 \times 10^{-34} \mathrm{J} \cdot \mathrm{s}\)