Chapter 27. Radius of a nucleus (27-1)
Question
x/NEfmm6prsETQ0ro4eJuRgGnycUpNTx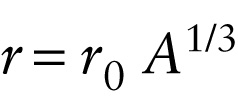
Question
U3+rIsjllisIa3NImPO6oNiaXAJpI30o09iRrAtWBmVRDokbh+KgfVfzaixbNRZRbBQ8QqG0dkh7gi2n394bDRkbxqkaPeG9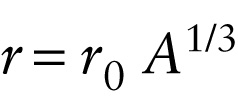
Question
j3XpClPlI5om0zNxzChS+v0rh/5xZhIJ4Jedzg==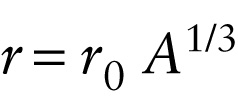
Review
The size of a nucleus (its radius and volume) is related to the mass number \(A\). Experiments show that all nuclei are approximately spherical and have radii proportional to the cube root of \(A\), or \(A^{1/3}\):
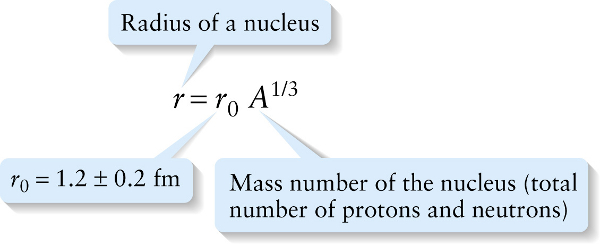
This equation states that if we increase the number of nucleons in a nucleus by a factor of 10, say from \(A = 20\) to \(A = 200\), the radius of the nucleus will increase by a factor of \(10^{1/3}= 2.2\) (Figure 27-3).