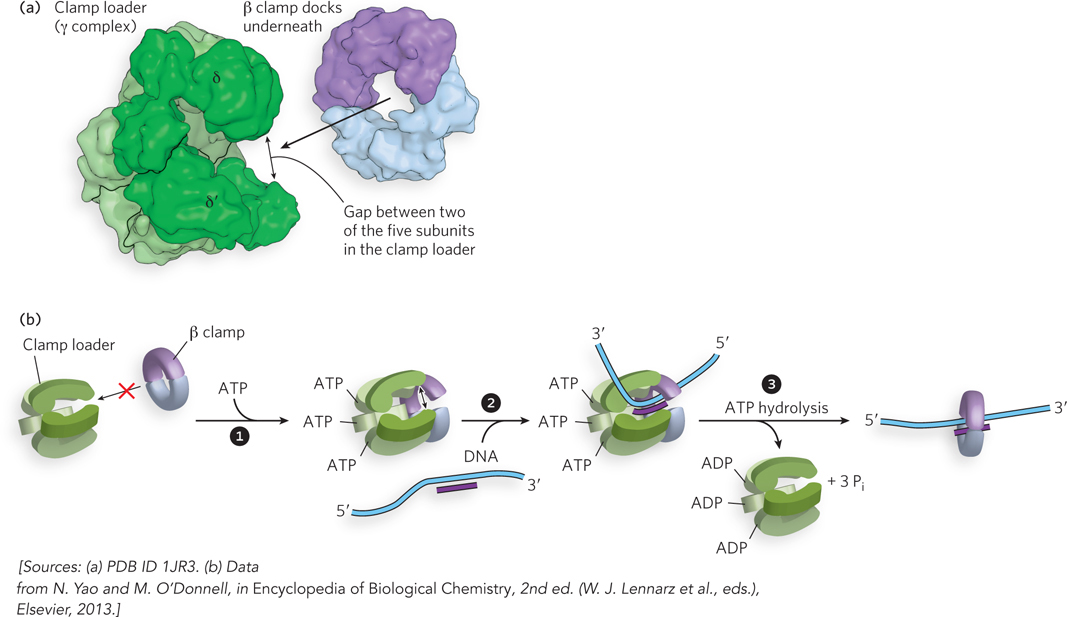
The E. coli clamp loader. The γ complex is shown here. (a) The five clamp- 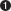 ATP binding to the γ subunits powers a conformational change that enables the binding and opening of the β clamp.
ATP binding to the γ subunits powers a conformational change that enables the binding and opening of the β clamp. 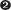 The combined γ comple
The combined γ complex– P– e- 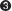 ATP hydrolysis ejects the clamp loader, allowing β to close again around the DNA.
ATP hydrolysis ejects the clamp loader, allowing β to close again around the DNA.
 ATP binding to the γ subunits powers a conformational change that enables the binding and opening of the β clamp.
ATP binding to the γ subunits powers a conformational change that enables the binding and opening of the β clamp.  The combined γ comple
The combined γ comple ATP hydrolysis ejects the clamp loader, allowing β to close again around the DNA.
ATP hydrolysis ejects the clamp loader, allowing β to close again around the DNA.