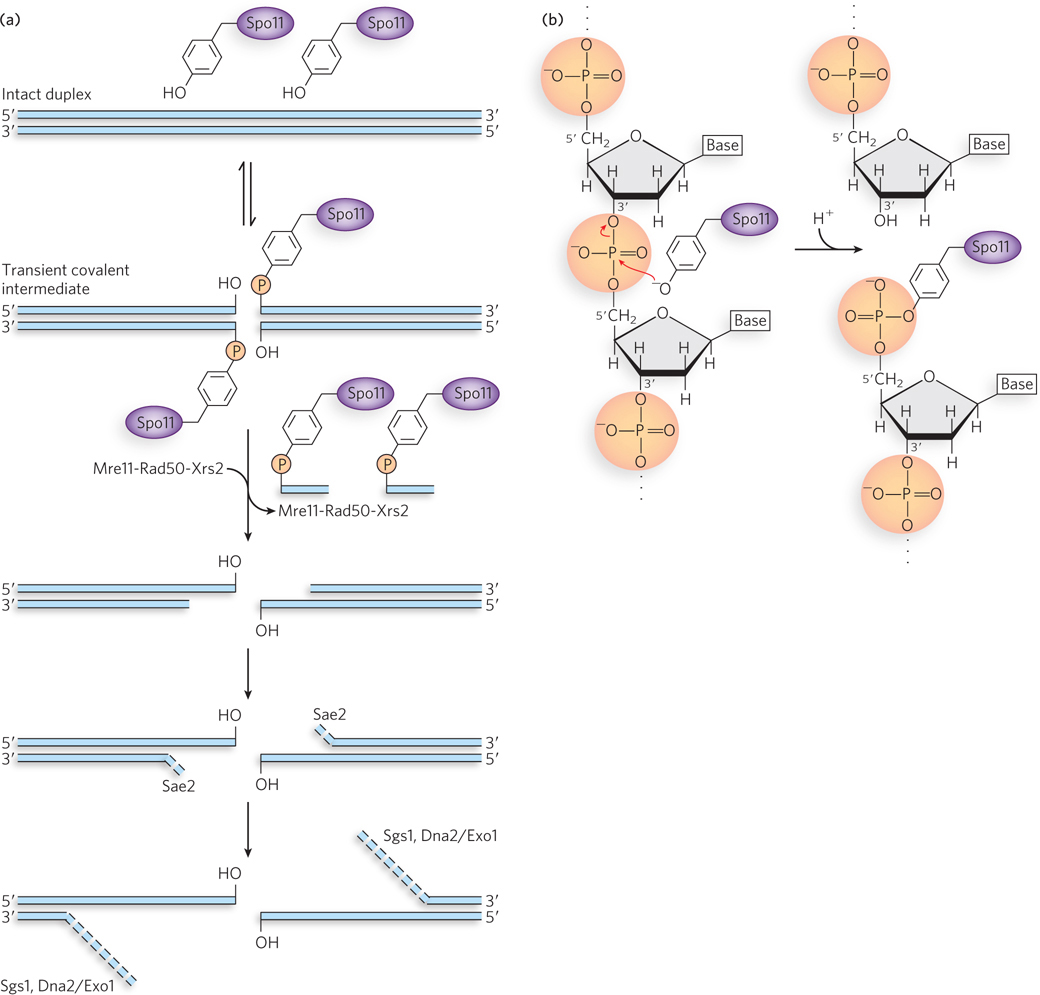
The Spo11 protein and initiation of meiotic recombination. (a) The reaction promoted by the Spo11 protein and subsequent processing of the double- e- l- 1- 0-