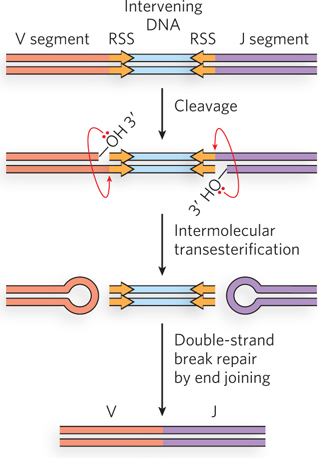
Immunoglobulin gene rearrangement. Proteins RAG1 and RAG2 bind to RSS (recombination signal sequences) and cleave one DNA strand between the RSS and the V (or J) segments that are to be joined. The liberated 3′ hydroxyl acts as a nucleophile, attacking a phosphodiester bond in the other strand to create a double- d- e- e-