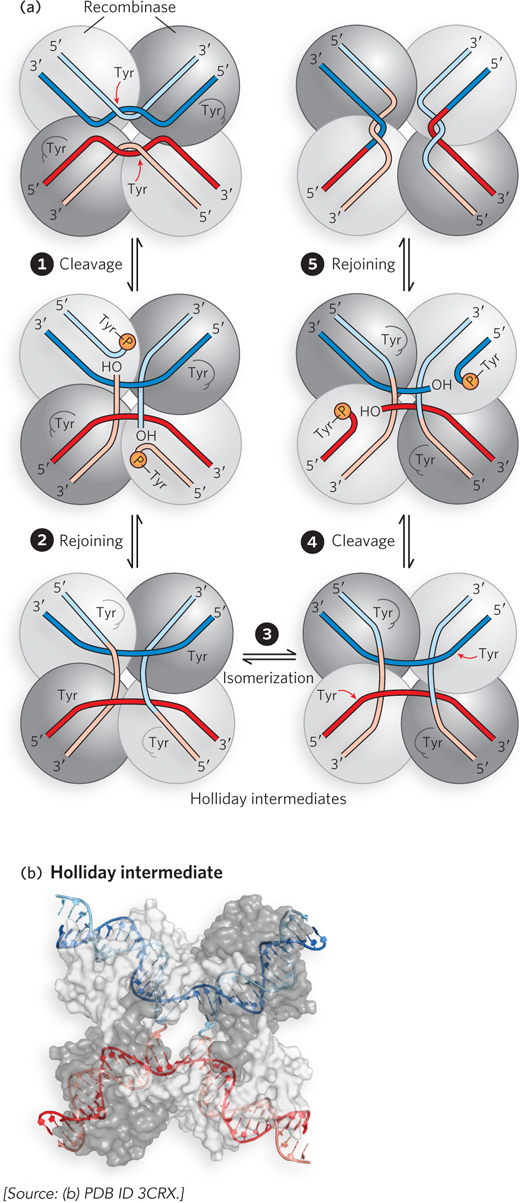
A site- e- e- e-