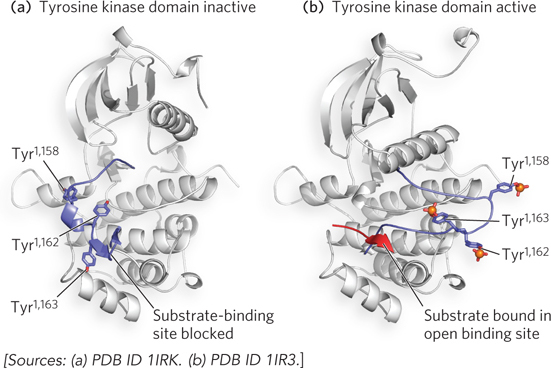
FIGURE 1 The tyrosine kinase domain of the insulin receptor is activated through autophosphorylation. (a) When the tyrosine kinase domain is inactive, the activation loop (blue) sits in the active site and none of the critical Tyr residues are phosphorylated. (b) When insulin binds the receptor, the tyrosine kinase activity phosphorylates Tyr1,158, Tyr1,162, and Tyr1,163. Introducing these three phosphate groups (shown in orange) results in a 30 Å movement in the activation loop, shifting it out of the substrate-