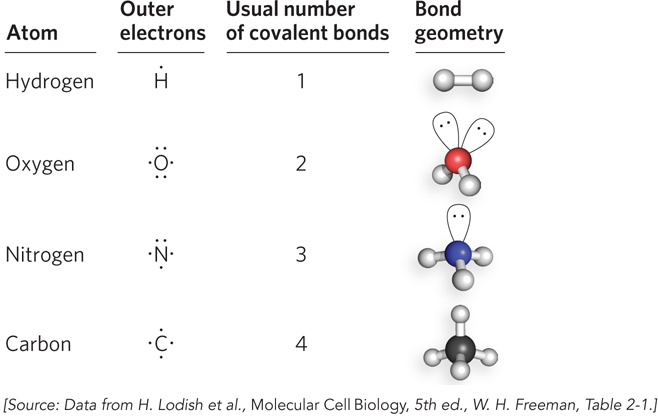
Valences of atoms that are common in biological molecules. Conventional representations of atoms known as Lewis structures (named for the chemist Gilbert Lewis) show the lone pairs of electrons— s—