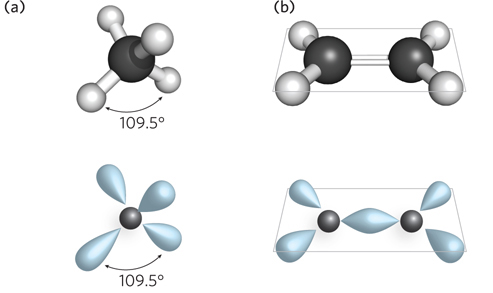
Geometry of single and double- e- l- d- e-