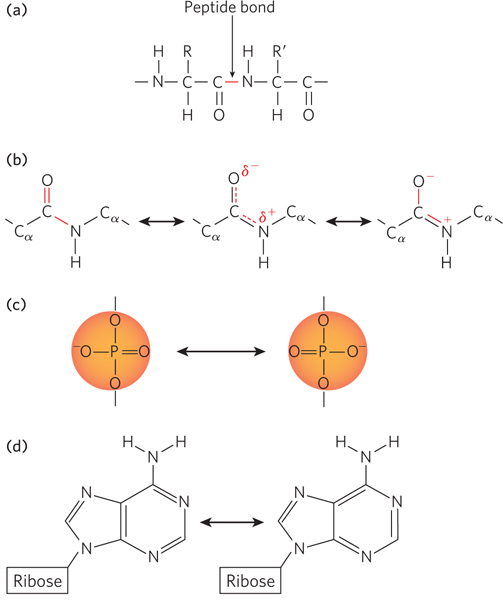
Resonance in peptides and nucleic acids. (a) A peptide bond covalently links the carboxyl group and amino group of adjacent amino acid residues in a protein. (b) Resonance between the resulting carbonyl and imino bonds gives each the properties of both a single and a double bond. Rotation is restricted about these bonds, and thus the attached chemical groups must lie in the same plane. Although the N atom in a peptide bond is often represented with a partial positive charge, as here, a careful consideration of bond orbitals and quantum mechanics indicates that the N has a net charge that is neutral or slightly negative. The partial positive and negative charges are represented by δ+ and δ−. (c) Resonance in the phosphate group (of the phosphodiester bond) of nucleic acids. (d) Resonance in the bases of nucleic acids; the adenosine nucleoside is shown here. See Chapter 6 for resonance structures of other bases.