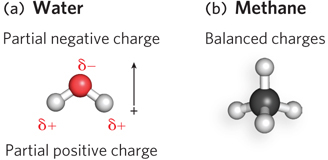
Polar and nonpolar molecules. (a) The polar water molecule has positive and negative poles and carries an electric dipole moment. Dipole moment is a vector quantity and is represented by a small arrow pointing from the positive charge toward the negative charge. (b) A nonpolar methane molecule has no separation of charges.