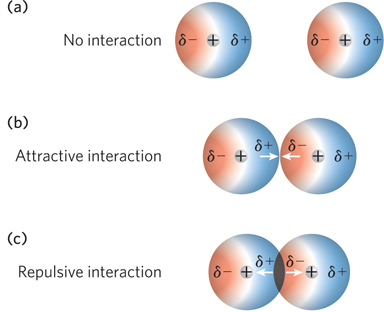
Attractive and repulsive van der Waals interactions. (a) Atoms that are farther apart than the sum of their van der Waals radii do not experience van der Waals forces. (b) Attractive interactions arise when atoms are separated by a distance equal to their combined van der Waals radii. (c) Repulsive interactions arise when atoms are closer than their combined van der Waals radii.