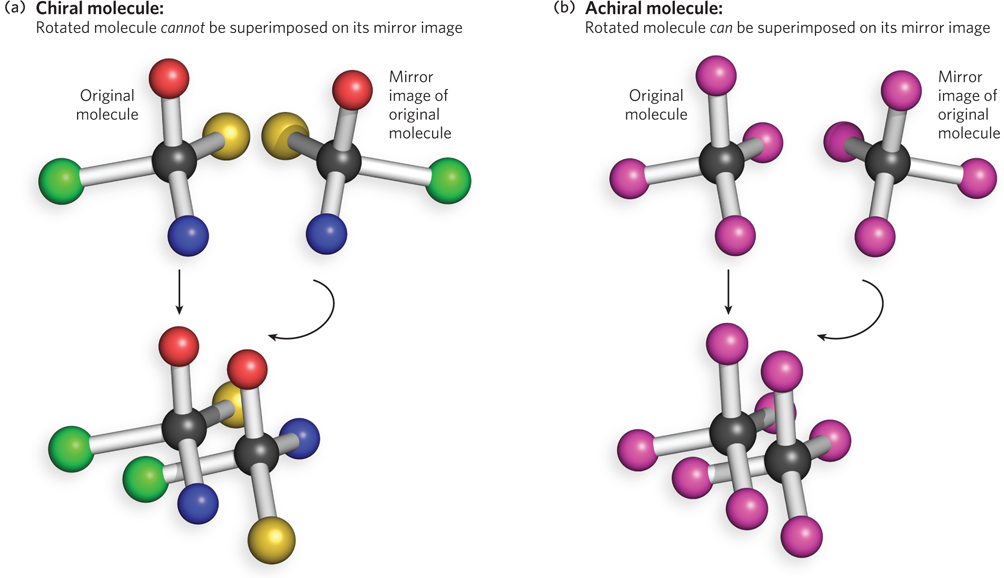
Chiral versus achiral molecules. (a) A molecule is chiral if it is not identical to its mirror image (i.e., it cannot be superposed on its image). When bonded to four atoms or functional groups that are not identical to each other, a carbon atom is chiral, because the four atoms or functional groups can be arranged in two different ways that are not superposable mirror images. All four of the bonded groups must be different from each other for the carbon center to be chiral. Thus, the two forms of the molecule have the same chemical formula but different chemical behavior. (b) In contrast, a carbon atom bonded to four identical atoms is achiral, because only one configuration is possible and any arrangement of the four bound atoms is superposable on its mirror image.