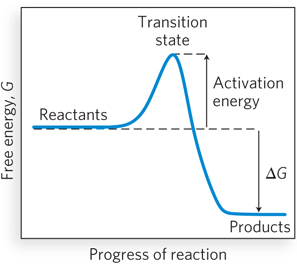
The activation barrier between reaction substrates and products. The starting and ending points of chemical reactions are bridged by an energy barrier, called the activation energy, that separates the reactants from the products. If the difference in energy between the products and the transition state is greater than the difference in energy between the reactants and the transition state, then at equilibrium the amount of product will be greater than the amount of reactant, because the energy barrier to the reverse reaction is higher. The difference in free energy (ΔG) between the reactants and products is negative in the forward reaction, indicating that this reaction is energetically favorable. This type of diagram is called a reaction coordinate diagram (see Chapter 5).