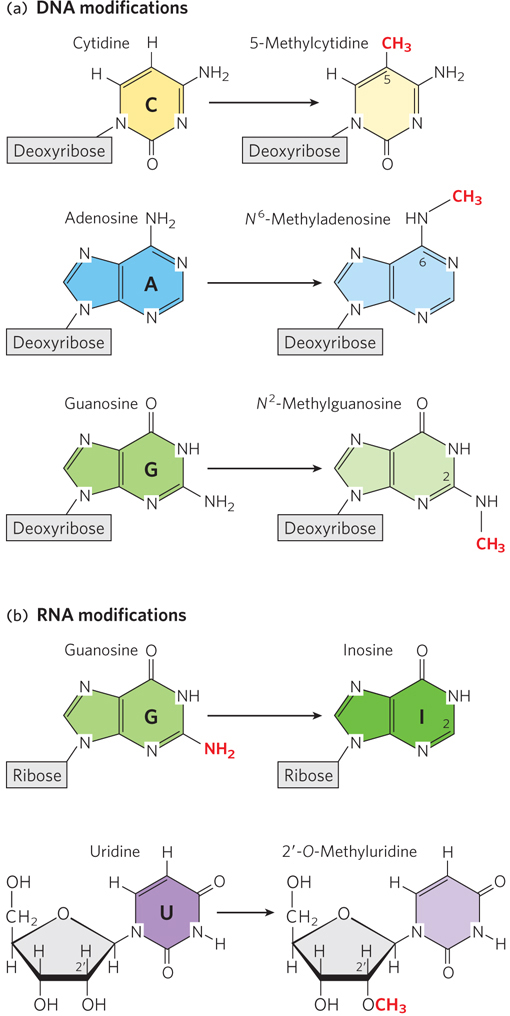
Chemical modification of nucleotide sugars and bases. (a) Examples of methylation modifications in DNA nucleotides. The extra methyl group on 5-