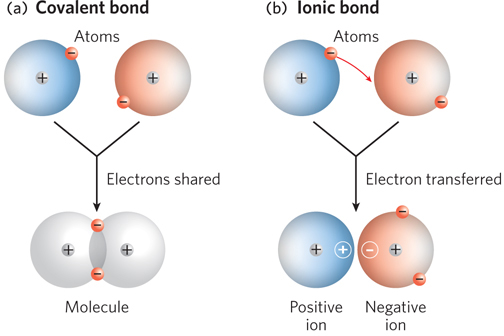
Covalent bonds and ionic bonds compared. (a) A covalent bond forms when two atoms share electrons so that their outer electron shells are filled. (b) An ionic bond forms when one or more electrons are completely transferred from one atom to another, such that one atom bears a positive charge, and the other a negative charge. Note the space between the atoms paired in an ionic bond; they are not as close together as atoms joined by a covalent bond.