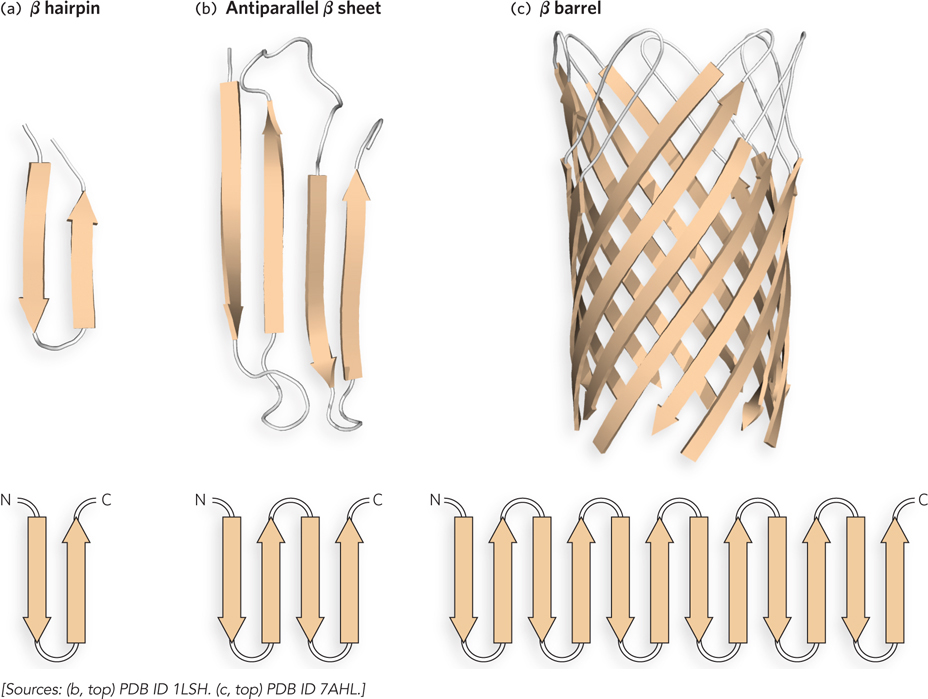
Supersecondary structures of antiparallel β sheets. (a) The β hairpin motif is an antiparallel β sheet composed of two β strands adjacent in the primary structure; the strands are often connected by β or γ turns. (b) The β strands of antiparallel (shown here) and parallel β sheets tend to have a right- e- p- d-