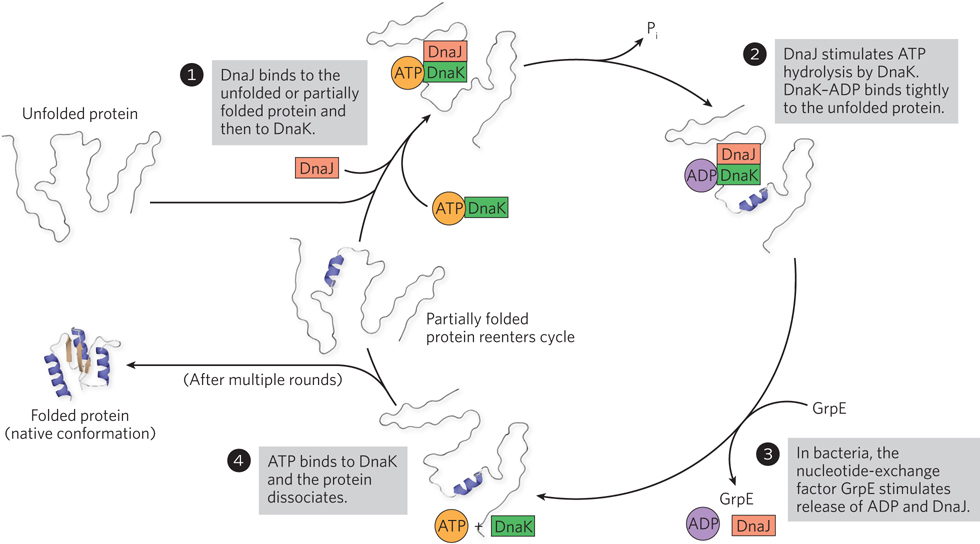
Chaperone-