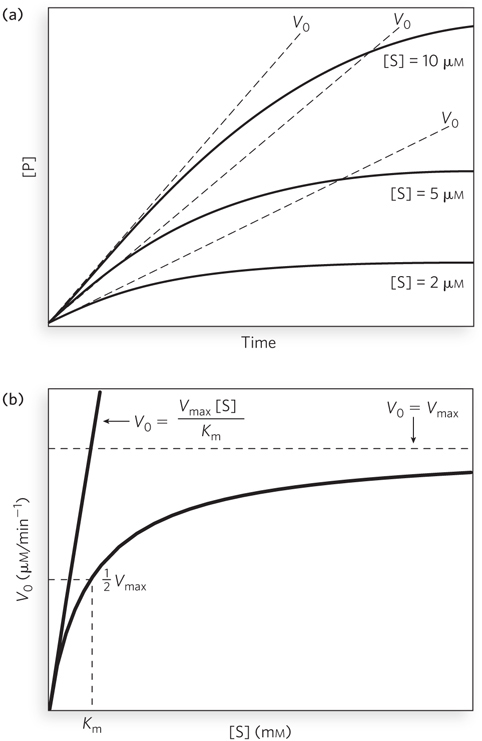
The initial velocity of an enzyme- e-