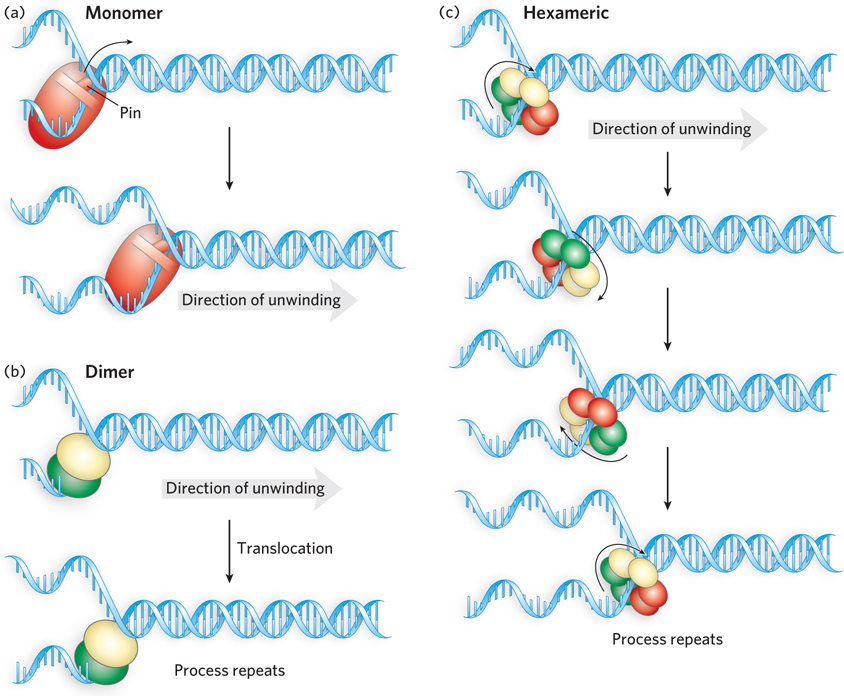
Unwinding reaction mechanisms for DNA helicases. (a) A monomeric helicase hydrolyzes ATP, causing conformational changes that couple unidirectional DNA translocation with destabilization of the duplex. Some of these enzymes have pinlike structures that function in strand separation. (b) A functional dimer model. A dimeric helicase has two identical subunits. These may alternate in binding to single- e-